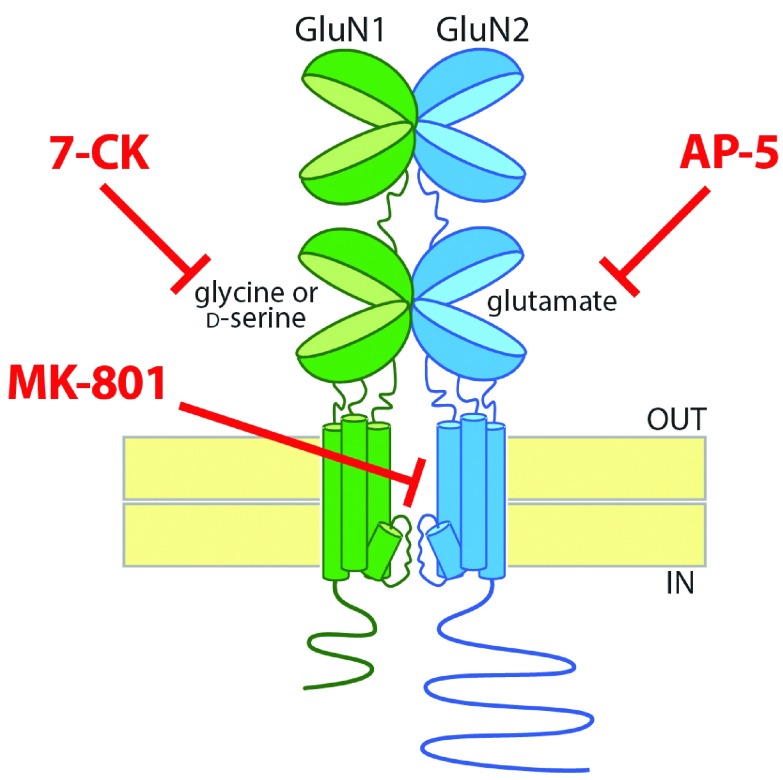
Figure 1. Pharmacology of the N-methyl- d-aspartate receptor (NMDAR).
Most NMDARs are tetrameric proteins containing two GluN1 and two GluN2 subunits (for clarity, only one of each is pictured). For the NMDAR channel to open, both glutamate and a co-agonist, which can be glycine or d-serine, need to bind to clamshell-like ligand-binding domains on the GluN2 and GluN1 subunits, respectively. There are multiple approaches to block ion flow through the NMDAR channel: a competitive antagonist for the glutamate-binding site on the GluN2 subunits (e.g. AP-5), a competitive antagonist for the glycine/ d-serine binding site on the GluN1 subunits (e.g. 7-CK), or an uncompetitive blocker of the channel itself (e.g. MK-801). 7-CK, 7-chlorokynurenic acid; AP-5, (2R)-amino-5-phosphonovaleric acid; MK-801, dizocilpine.