Figure 1.
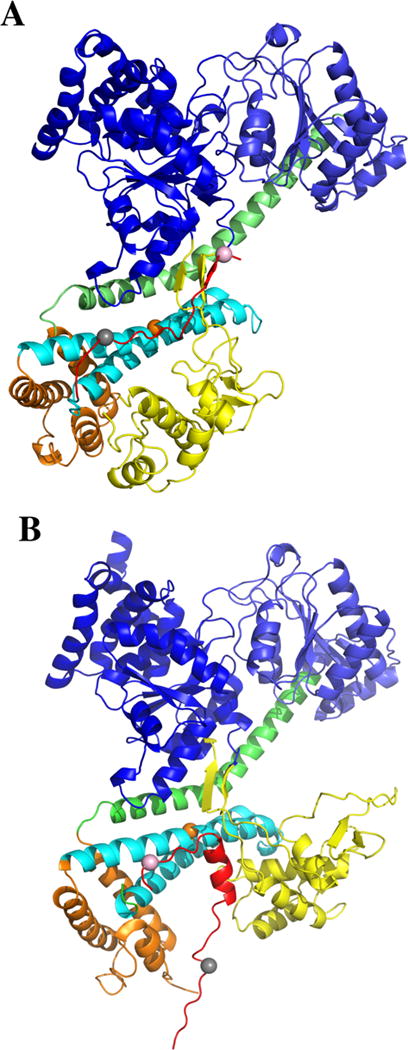
Comparison of SecA signal peptide-binding parallel and perpendicular models. (A) A model where the PhoA signal peptide is bound mainly in a parallel fashion to the THF subdomain of bsSecA is compared to (B) a model where the KRRLamB signal peptide is bound mainly in a perpendicular fashion to the THF subdomain of bsSecA. In B, the ecSecA portion of the peptide bound complex from the NMR structure (PDB ID: 2VDA) was replaced with bsSecA (PDB ID: 1M6N) by superimposing and aligning the major structural domains to create a KRRlamB signal peptide bound bsSecA complex. Color coding of the SecA domains or subdomains is as follows: NBD-I (dark blue), NBD-2 (light blue), PPXD (yellow), HSD excluding THF (green), THF (cyan), and HWD (brown). The bsSecA Trp724 residue is depicted as an orange space-filling sphere. (A) PhoA signal peptide (modeled as the CTL domain of SecA) or (B) KRRLamB signal peptide is shown in red with dye labeled residues at the beginning or end of the corresponding signal peptide depicted by gray or pink space-filling spheres, respectively.
