Abstract
Background and Objectives
Stimuli that are repeatedly paired with substance use, such as drug paraphernalia, can themselves elicit drug craving. The aim of this study was to examine whether particular cue types elicit greater craving responses than others among individuals with opioid dependence.
Methods
Participants seeking inpatient treatment for opioid dependence were recruited for a study of cue-induced craving. This sample (N=50), included 25 primary heroin users, 20 primary prescription opioid users, and 5 users of heroin and prescription opioids equally. Participants completed a cue reactivity task, in which images of drug-related stimuli were presented on a computer screen, each followed by a question assessing state drug craving.
Results
Overall, participants reported higher craving following paraphernalia stimuli relative to drug stimuli. However, this was moderated by opioid type; there was significantly higher craving in response to images of paraphernalia cues in the heroin group, and higher craving in response to drug cues in the prescription opioid group.
Discussion and Conclusions
These findings highlight potential differences in cue reactivity to opioid paraphernalia and drug cues, which appears to be moderated by drug type.
Scientific Significance
Cue-induced craving is an important factor in relapse. This study adds further to the literature on cue-induced craving in opioid dependence, suggesting that craving may vary based on both cue type and opioid type. Future studies designed to discriminate the impact of substance of abuse, route of administration, and cue type will help to further understand cue-induced craving in this population.
Keywords: craving, opioid dependence, prescription opioids, drug cues, paraphernalia
Introduction
Stimuli that are repeatedly paired with drug use often acquire rewarding properties and become conditioned cues that independently elicit craving for the drug.1, 2 Among those with substance use disorders, drug use often occurs after exposure to these conditioned cues,3 and individuals with greater cue-induced craving are at a higher risk for relapse following treatment.4, 5 Environmental contexts (e.g., the settings in which drugs are used) appear to elicit less craving relative to more proximal cues, such as drugs and paraphernalia.6 This difference between proximal and distal cues likely is mediated by the strength of associative learning, which may vary based on the frequency and proximity of the pairing of the cue and drug use. However, few studies have attempted to measure and distinguish craving among different cue types. Examining differences in craving to different types of cues may both enhance our understanding of associative learning in substance use disorders, and inform treatments that aim to reduce the impact of cue-induced craving on substance use and relapse.
Images of drugs and paraphernalia (e.g., needles, lighters) elicit reliable craving responses in those diagnosed with substance use disorders, such as cocaine-dependent individuals,7 cigarette smokers,8 heroin-dependent individuals,9 alcohol-dependent patients,10 and marijuana users.11 Similarly, tactile handling of drug-related paraphernalia is associated with craving when compared to handling neutral objects. 7, 12–14 Importantly, however, we are not aware of any previously published papers that directly compare subjective craving elicited by drug cues relative to paraphernalia cues.
Differences in cue-induced craving may be observed both within a particular drug category (e.g., needles vs. heroin powder in heroin users) and also across drug types. Opioid analgesics and heroin may differ with respect to the strength of associative learning between cues and use. Because stimuli associated with prescription drug use (e.g., pills, pill bottles) are commonly encountered outside of a drug-using context, the strength of learning between these cues and drug use may be weaker than associations formed between more distinctly linked stimuli (e.g., needles and heroin). In a prior study, our group examined differences in cue-induced craving to drug images relative to neutral images among inpatients with opioid dependence, who were either primary heroin or prescription opioid users.15 In this study, we found that both groups reported strong and significant craving in response to drug stimuli relative to neutral stimuli, and that this association was larger among those dependent upon heroin.
The current study builds upon our prior study to investigate whether images of opioids and opioid paraphernalia produce differential subjective craving responses in prescription opioid- and heroin-dependent individuals. We examined craving responses to images of opioids and opioid paraphernalia in a treatment-seeking sample of individuals dependent upon heroin, prescription opioids, or both.
2. Methods
2.1 Participants
We recruited a sample of 52 adults (11 women) receiving treatment for opioid dependence from the inpatient substance use disorder treatment unit of a private psychiatric hospital for a study of cue-induced craving. Opioid dependence diagnosis was based on the criteria delineated in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition.16 Patients who did not exhibit significant withdrawal symptoms or other acute symptoms (e.g., severe psychotic symptoms) that would preclude engaging in study tasks were eligible to participate. These criteria were determined based on review of the medical chart, consultation with clinical staff on patients' ability to complete study procedures, and completion of a measure of withdrawal symptoms. Two participants were excluded from the data analysis due to failure to complete study procedures or to adhere to task instructions. Thus, a sample of 50 participants (10 women) was used for this analysis.
Participants reported a mean age of 26.8 years (SD = 8.3). The sample was predominantly Caucasian (86%) and non-Hispanic (82%), although some participants declined to report race (n = 2) or ethnicity (n = 7). Most participants reported some college education or more (66%); 24% reported high school education or equivalent (24%) and 10% less than a high school education. When asked about primary drug of abuse, 50% (n = 25) of participants reported heroin, 40% reported prescription opioids (n = 20), and 10% (n = 5) reported using both equally.
All participants required medical detoxification from opioids. The mean days of prescription opioid use in the 30 days prior to admission in the prescription opioid users was 29.30 (SD = 9.71), and the mean days of heroin use in the heroin group was 26.28 (SD = 7.28). The mixed opioid group reported an average of 22.00 (SD = 9.19) days of heroin use and 16.20 (SD = 11.17) days of prescription opioid use. The most common route of administration of prescription opioids among the prescription opioid users was intranasal (90%). Intranasal and injection use were the most common routes of heroin administration (84%) in the heroin group. Oxycodone was the most frequently reported primary prescription opioid of abuse (85% in the prescription opioid group).
2.2. Procedures
After providing informed consent, a member of the study staff administered the Clinical Opioid Withdrawal Scale 17 to confirm that the participant was below the clinical threshold (<4) for minor withdrawal before initiating study procedures. Participants then completed a brief battery of self-report measures and a cue-induced craving task. Participants were provided with a $20 gift card to a local vendor for their participation. All study procedures were approved by the local institutional review board.
Cue reactivity was assessed using self-reported craving rated during a computer-based presentation of pictorial stimuli. Each participant was presented with 90 distinct images, which consisted of 40 heroin-related (e.g., needles, powder), 40 prescription opioid-related (e.g., crushed pills, pill bottles), and 10 neutral (e.g., pencils, sugar packets) photographs. All images were developed for the purpose of the parent study, and were taken with the same background (a wooden table). Only heroin- and prescription opioid-related images were used for this study. These stimuli included images of a variety of materials designed to best approximate pertinent opioid cues. These images included variations of various colors of powder, presented in both a small pile or in lines; various shapes, colors and sizes of pills, including crushed pills; prescription pill bottles; small bags of powder; needles and other injection stimuli (e.g., spoons, cotton); and rolled papers for intranasal administration. Some images included a hand (e.g., pills in a hand) or arm (held out next to a needle). For this paper, any stimuli that included a combination of paraphernalia and drug (e.g., powder and a needle) were excluded to allow the examination of paraphernalia vs. drug exclusively. These images were presented in a randomized order for each participant, using EPrime Version 2.0.
After the presentation of each cue, participants rated their craving using a 3-item rating scale of state drug craving adapted from a validated craving measure.15, 18 For the purpose of this analysis, the first item (“How much do you have an urge or craving for opioids right now?”) was used as a measure of state opioid craving. The possible scores for this item ranged from 0-10.
2.3. Data Analysis
For the cue-induced craving task, missing values (1.6% of all responses), invalid responses (0.2%), and response times of less than 250ms (0.2%) were excluded from analysis. Self-reported opioid craving following each stimulus presentation was averaged for images categorized as "drug" vs. "paraphernalia" stimuli. This categorization was done by study investigators. Given both potential overlap between stimuli that may be salient for prescription opioid vs. heroin users (e.g., powder), as well as potential distinctions (e.g., crushed pills), we selected stimuli that optimized the mean reported cue salience for each group. Specifically, mean salience scores were calculated separately for the heroin and prescription opioid users; the most salient cues (with a minimum of a moderate level of craving) were used for this analysis. This consisted of the 24 most salient images, counterbalanced across both group (prescription opioid vs. heroin) and stimulus type (paraphernalia vs. drug). The heroin images rated as most salient included images of a small pile of fine brown powder, an image of a small pile of white powder with two "lines", and images of injection stimuli. The prescription opioid images rated as most salient included: images of lines of white powder, white powder in combination with small white pills, small round white pills in a hand, and images of a prescription bottles in a hand and on a table. Cue salience was assessed using a single item "How much did the picture remind you of times that you have used opioids?". This was done to ensure that the images selected were salient for each group.
Repeated-measures ANOVA was then used to examine within-subjects differences between craving to drug vs. paraphernalia stimuli, controlling for primary opioid of abuse. All 50 participants who completed the study were included in this analysis, with the dependent variable of craving for the primary drug of abuse. This analysis was adequately powered (.80) to detect a medium effect size (f=.20) for the within-subjects comparison.
3. Results
The heroin and prescription opioid groups did not differ with respect to any sociodemographic variables collected (age, gender, education, race, or ethnicity). Collapsing across all groups (primary heroin users, primary prescription opioid users, and equal users of both), there was a significant within-subjects difference between paraphernalia and drug stimuli, characterized by greater craving for drug cues (F[1,46] = 5.45, p = .02). There was also an interaction between primary opioid and craving (F[2,46] = 21.80, p < .001).
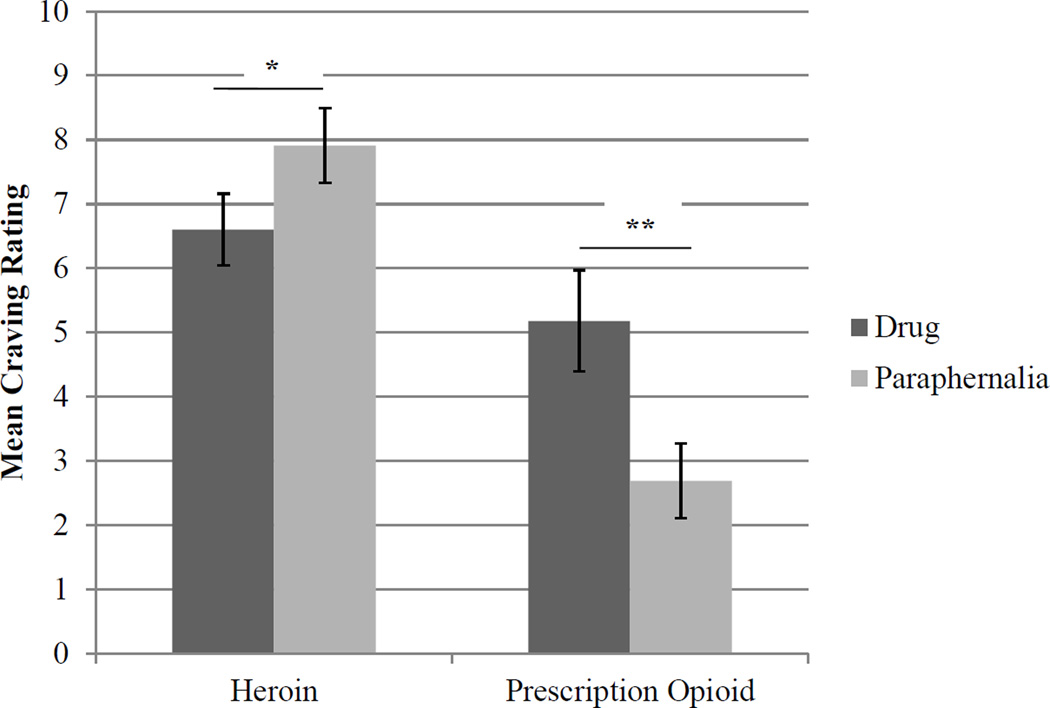
These results suggested that the difference in craving between paraphernalia and drug stimuli varied based on the primary opioid of abuse. Post-hoc paired-samples t-tests indicated that in the heroin group, craving for paraphernalia was significantly higher than for drug images (mean difference = -1.31, t[24] = -4.50, p = .001). In contrast, in the prescription opioid group, craving for drug cues was significantly higher than craving for paraphernalia (mean difference = 2.48, t[18] = 5.63, p < .001). See Figure 1.
Figure 1.
Craving in Response to Drug vs. Paraphernalia Stimuli in Primary Prescription Opioid vs. Primary Heroin Users
Note. Craving ratings reflect craving for stimuli rated as moderately salient or higher on average by participants in the heroin and prescription opioid group separately. *p < .01, **p < .001.
The majority (92%) of heroin users reported lifetime use of prescription opioids, with 42% reporting some use in the past 30 days. Thus, this subgroup provides the opportunity to examine whether the difference between the heroin and prescription opioid groups was reflective of a difference between groups or between the stimuli. Among primary heroin users who also reported a lifetime history of prescription opioid use (n=23), craving for prescription opioid drug cues was higher than for paraphernalia cues (mean difference = 1.90, t[22]=3.99, p = .001). Of note, because prescription opioid injection was rare in this sample, the prescription drug paraphernalia cues that were rated as most salient did not include injection stimuli. Thus, this effect may be attributable to differences in route of administration.
4. Discussion
In a sample of participants receiving inpatient treatment for opioid dependence, differences in cue-induced craving to paraphernalia cues relative to drug cues was moderated by the type of opioid. Participants primarily using heroin reported significantly higher craving to heroin paraphernalia stimuli relative to heroin drug stimuli. Among those who primarily used prescription opioids, however, there was significantly higher craving for drug stimuli relative to paraphernalia stimuli.
There are several potential reasons for this difference in cue-induced craving. First, the majority of the heroin users were injection users (84%), whereas only a minority of prescription opioid users reported injection use (10%). Accordingly, images of needles were not rated as highly salient by the prescription opioid group. Thus, this difference in perception of salience is likely based on predominant route of administration. Prescription bottles and pills are often encountered in various generalizable contexts (e.g., doctors’ offices, homes, convenience stores with pharmacies), whereas needles and other stimuli specifically involved in heroin preparation are less likely to be encountered outside of the context of heroin use. Thus, image cues associated with injection use (e.g., needles) may be more salient than those associated with general use (e.g., pill bottles) or with intranasal use (e.g., rolled up paper). Taken together, these opioid-related imagery data suggest that cues that are more proximal to preparation and use elicit stronger craving. Nonetheless, replication of this finding, particularly with inclusion of a broader array of paraphernalia cues (e.g., paraphernalia for crushing pills), is needed to better understand differences in drug cue-induced craving between heroin- and prescription analgesic-using populations.
These findings have implications for clinical practice because of the importance of modifying behavioral responses to triggers. When considering relevant triggers for use, commonly utilized paraphernalia should be assessed in heroin users, with efforts to either limit access to these objects (e.g., by removing from one's home), and/or build skills to tolerate the craving elicited during exposure to these objects. Moreover, continued advances in understanding cue reactivity have shown promise for enhancing cue exposure techniques as a treatment strategy for those with substance use disorders. 19, 20 Continued understanding of the associative learning process in this population may be used to inform and enhance exposure-based approaches to reducing cue-induced craving.
There are several limitations to the current study. First, because there were no available previously validated stimuli in the literature for measuring response to prescription opioid use, we created our own stimulus set. Although these stimuli have demonstrated high levels of salience and craving relative to matched neutral cues,15 pertinent cues (e.g., certain types of paraphernalia) and ideographic cues (e.g., differences among prescription opioid pills based on manufacturer) may have been missed. Replication of these findings and establishment of the range of relevant cues in this population is needed. Second, the study sample was comprised of inpatients; we cannot assume that these findings will generalize to less severe or acute patient populations. There was some prescription opioid use in the heroin group, and vice versa, suggesting that there were few exclusive users of either opioid type. However, given that exclusive use of either opioid type is increasingly uncommon, prior studies of prescription opioid dependent populations have similarly allowed for some heroin use in this group.21 Moreover, we selected stimuli for this study based on those rated as most salient by each group to maximize craving response. Finally, because this was a secondary data analysis, the study stimuli were not specifically designed to test differences between paraphernalia and drug cues. However, stimuli were counterbalanced to include an equivalent number of stimuli with drug vs. paraphernalia images, somewhat mitigating this concern.
This study found that paraphernalia cues elicited greater craving that drug cues among heroin users, and that drug cues elicited greater craving among prescription opioid users in an opioid-dependent sample. Future studies aimed to better understand this difference will aid in both the understanding of associative learning in opioid dependence and in the enhancement of treatments to improve resilience to triggering cues in this population. In particular, future studies that aim to dissociate the differences related to route of administration vs. drug type will be important to understanding differences in craving in this population.
Acknowledgments
Funding for effort on this project was provided by the following sources: the Harvard Medical School Livingston Award (McLean Hospital, Belmont MA; PI: Dr. McHugh), and NIDA grants K23 DA035297 (McLean Hospital, Belmont MA; PI: Dr. McHugh), K01 DA034028 (McLean Hospital, Belmont MA; PI: Dr. Mashhoon), U10 DA015831, and K24 DA022288 (McLean Hospital, Belmont MA; PI: Dr. Weiss).
Footnotes
Declaration of Interest
Dr. Weiss has consulted to Reckitt Benckiser and Titan Pharmaceuticals. All other authors declare they have no financial relationships with commercial interests. The authors alone are responsible for the content and writing of this paper.
References
- 1.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- 2.O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 3.Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatseas M, Denis C, Massida Z, Verger M, Franques-Reneric P, Auriacombe M. Cue-induced reactivity, cortisol response and substance use outcome in treated heroin dependent individuals. Biol Psychiatry. 2011;70:720–727. doi: 10.1016/j.biopsych.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ. Proximal versus distal cues to smoke: the effects of environments on smokers' cue-reactivity. Exp Clin Psychopharmacol. 2008;16:207–214. doi: 10.1037/1064-1297.16.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reid MS, Mickalian JD, Delucchi KL, Berger SP. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology. 1999;20:297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- 8.Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: physiological and self-report manifestations. J Abnorm Psychol. 1997;106:15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- 9.Yu J, Zhang S, Epstein DH, et al. Gender and stimulus difference in cue-induced responses in abstinent heroin users. Pharmacol Biochem Behav. 2007;86:485–492. doi: 10.1016/j.pbb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Nees F, Diener C, Smolka MN, Flor H. The role of context in the processing of alcohol-relevant cues. Addict Biol. 2012;17:441–451. doi: 10.1111/j.1369-1600.2011.00347.x. [DOI] [PubMed] [Google Scholar]
- 11.Bordnick PS, Copp HL, Traylor A, et al. Reactivity to cannabis cues in virtual reality environments. J Psychoactive Drugs. 2009;41:105–112. doi: 10.1080/02791072.2009.10399903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrman RN, Robbins SJ, Childress AR, O'Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- 13.LaRowe SD, Saladin ME, Carpenter MJ, Upadhyaya HP. Reactivity to nicotine cues over repeated cue reactivity sessions. Addict Behav. 2007;32:2888–2899. doi: 10.1016/j.addbeh.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nickerson LD, Ravichandran C, Lundahl LH, et al. Cue reactivity in cannabis-dependent adolescents. Psychol Addict Behav. 2011;25:168–173. doi: 10.1037/a0021117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McHugh RK, Park S, Weiss RD. Cue-induced craving in dependence upon prescription opioids and heroin. Am J Addict. 2014;23:453–458. doi: 10.1111/j.1521-0391.2014.12129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 17.Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) J Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- 18.Weiss RD, Griffin ML, Hufford C. Craving in hospitalized cocaine abusers as a predictor of outcome. Am J Drug Alcohol Abuse. 1995;21:289–301. doi: 10.3109/00952999509002698. [DOI] [PubMed] [Google Scholar]
- 19.Saladin ME, Gray KM, McRae-Clark AL, et al. A double blind, placebo-controlled study of the effects of post-retrieval propranolol on reconsolidation of memory for craving and cue reactivity in cocaine dependent humans. Psychopharmacology (Berl) 2013;226:721–737. doi: 10.1007/s00213-013-3039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue YX, Luo YX, Wu P, et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336:241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68:1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]