Figure 2. Identification of C99‐Bpa interaction sites in γ‐secretase.
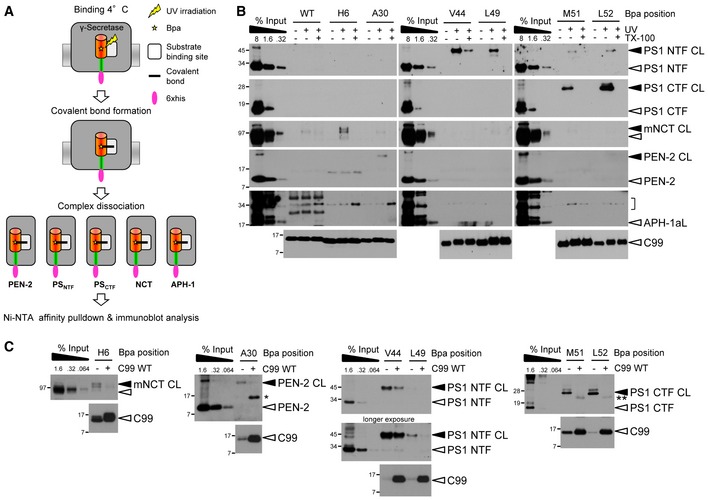
- Schematic representation of the substrate‐photocrosslinking strategy.
- Identification of C99 interaction sites with γ‐secretase. C99‐Bpa substrates were irradiated with UV light in the presence of CHAPSO‐solubilized γ‐secretase. Crosslinked substrates were captured by Ni‐NTA affinity pulldown and bound subunits were identified by a ˜10‐kDa molecular weight increase compared to the input. Results are shown for the major substrate crosslinking residues. Crosslink formation was not observed in the absence of UV irradiation or in the presence of Triton X‐100, which dissociates the γ‐secretase complex, proving crosslink specificity. Bracket indicates the molecular weight range of putative C99‐APH‐1 crosslink bands. APH‐1aL, long splice variant of APH‐1a.
- Excess amounts of parental C99 WT substrate compete crosslinking of C99‐Bpa substrates to γ‐secretase. Single and double asterisks indicate antibody crossreactivities to C99 monomer and dimer, respectively.
