Figure EV2. Additional pharmacological validation of substrate crosslinking to NCT and PEN‐2.
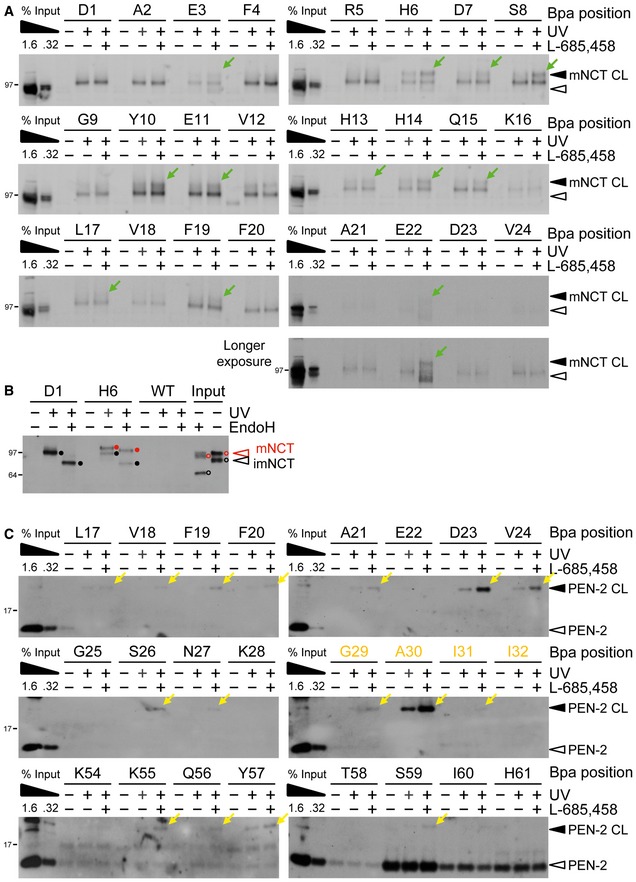
- Validation of functional relevance of C99‐Bpa interactions with NCT by the modulation of substrate crosslinking in the presence of L‐685,458. For a number of substrates binding to mature NCT was increased in the presence of L‐685,458, suggesting that the exclusion of substrate from the active site by the GSI caused substrate trapping at exosites in NCT. Residues for which crosslinking could not be detected even in the longest exposures on the films are not shown. Note that the crosslinking to immature NCT that was observed for all substrate residues did not change in the presence of L‐685,458, suggesting functionally irrelevant binding. See also (B). Green arrows indicate the residues for which increased labeling was observed.
- Deglycosylation by endoH revealed that the crosslink of H6 to NCT originated from binding to complex glycosylated mature NCT, while that of D1 from binding to immature NCT. Closed and open circles indicate partial endoH resistance of mature NCT (red) and endoH sensitivity of immature NCT (black) for the crosslinked samples or the uncrosslinked input control, respectively.
- Validation of functional relevance of C99‐Bpa interactions with PEN‐2 by the modulation of substrate crosslinking in the presence of L‐685,458. Increased substrate crosslinking in the presence of the active site‐targeting GSI is consistent with the presence of exosites in PEN‐2 and supports its role in substrate recruitment. Residues for which crosslinking could not be detected even in the longest exposures on the films are not shown. TMD residues are highlighted in orange. Yellow arrows indicate the residues for which increased labeling was observed.
