Figure 1.
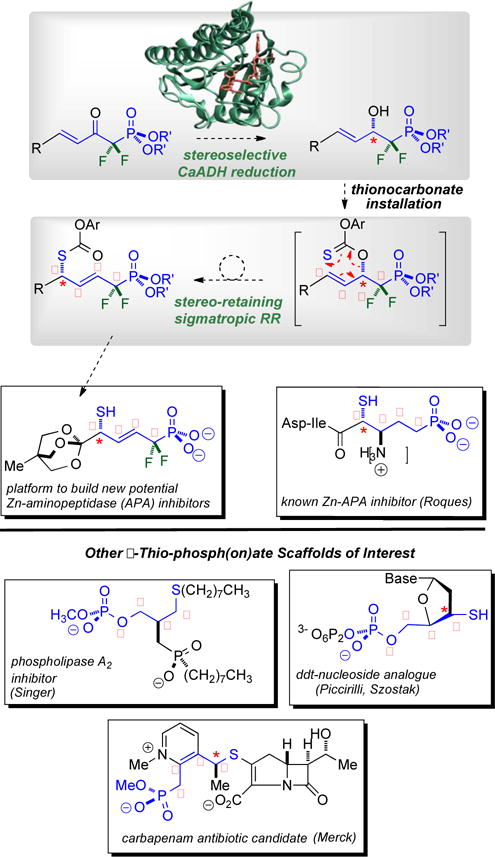
Stereocontrolled route into densely functionalized δ-thio-α,α-difluorophosphonates of interest to chemical biology. Top: CaADH is challenged with a new ketophosphonate substrate class to set the absolute stereochemistry, and a new [3,3]-sigmatropic RR is employed to parlay the enzymatically established β-C-O stereochemistry into the δ-C-S center; Bottom: Mapping the δ-thio-α,α-difluorophosphonate motif onto lipid, carbohydrate and β-lactam scaffolds of interest to chemical biology.
