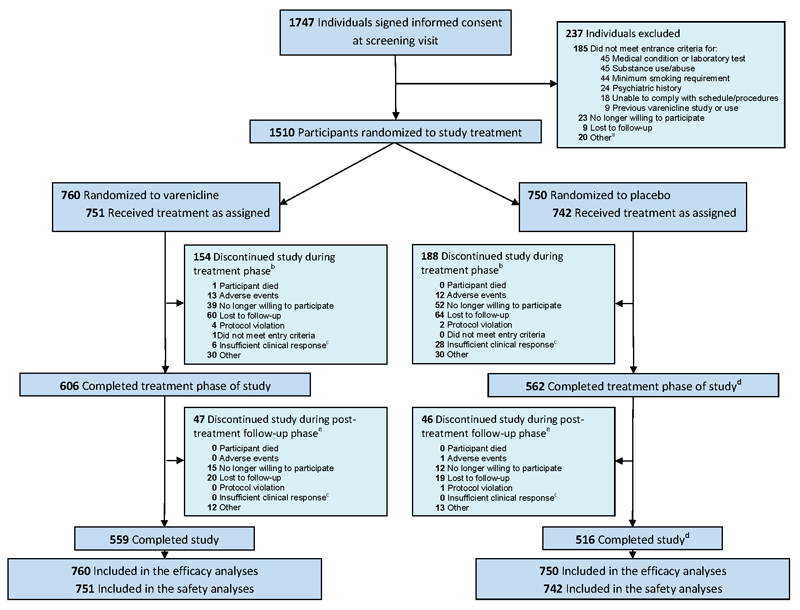
Figure 1.
CONSORT Diagram
aParticipants not randomized to study treatment due to reasons classified by the investigators as “other” included reasons such as: did not attend randomization visit; unable to commit to attending study visits; change in work schedule; change in concomitant medications; change in personal circumstances; and unavailability of urine drug screening kits. bTreatment phase was weeks 1 through 24. Includes 9 varenicline participants and 7 placebo participants who withdrew from the study before receiving study medication counted under the respective category for reasons of withdrawal. Note that one placebo participant stayed in the study although did not take any study medication, also see d. Discontinuations from study due to reasons classified by the investigators as “other” included reasons such as: new job or change in work schedule; moved out of area; change in personal or family circumstances; and unwilling or unable to attend visits. cInsufficient clinical response was a prepopulated option chosen by the investigators on the case report forms. dIncludes 1 placebo participant who did not receive any study medication but completed the study. ePost-treatment follow-up phase was weeks 25 through 52. Discontinuations from study due to reasons classified by the investigators as “other” included reasons such as: new job or change in work schedule; moved out of area; change in personal or family circumstances; and unwilling or unable to attend visits.