Fig 4.
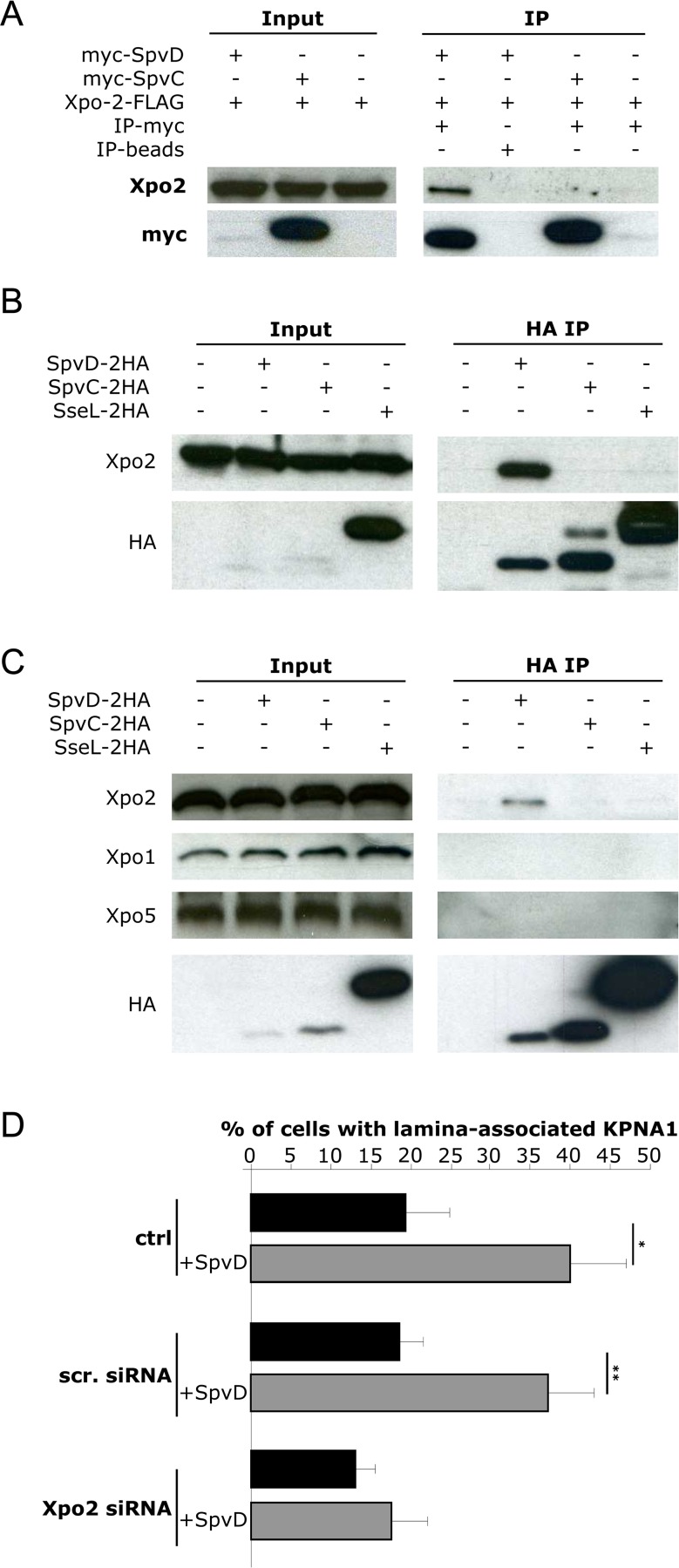
SpvD interacts with Xpo2 (A) HEK cells transfected with vectors expressing Xpo2-FLAG and myc-SpvD or myc-SpvC were lysed and proteins were immunoprecipitated with anti-myc antibody-conjugated beads, or beads as a control. Xpo2-FLAG, myc-SpvD and myc-SpvC were detected in input samples (input) and after immunoprecipitation (IP) by means of SDS-PAGE and immunoblotting. (B) RAW macrophages or (C) HeLa cells were infected for 20 h with wild-type Salmonella expressing either SpvD-2HA, SpvC-2HA or SseL-2HA. Proteasome inhibitor MG132 was added to the culture medium for the last 2 h of infection. Cells were then lysed and proteins were immunoprecipitated with HA antibody. SDS-PAGE blots were probed for HA and endogenous Xpo2, Xpo1 or Xpo5. (D) HeLa cells depleted of Xpo2 (Xpo2 siRNA) or treated with scramble siRNA (scr. siRNA) and then transfected with FLAG-KPNA1 and myc-SpvD were fixed and labelled with anti-myc and anti-FLAG antibodies. Intra-nuclear rings of KPNA1 were quantified blindly by microscopy. Values are expressed as mean ± SEM of 3 independent experiments and P-values were obtained using two-tailed unpaired Student's t-test (*p < 0.05; ** p < 0.01).