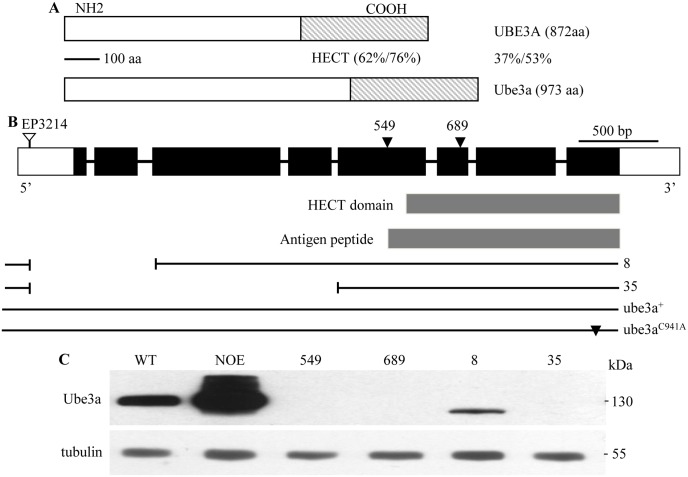
Fig 1. Characterization of ube3a mutations.
(A) The percent amino acid identity/similarity between Drosophila Ube3a and human UBE3A. (B) Intron-exon organization and various mutations of ube3a. Coding and non-coding exons are represented by black and empty rectangles, respectively. Triangles indicate nonsense mutations; the HECT domain and the peptide used for antibody production are indicated. Two deletions, ube3a8 and ube3a35, produced by imprecise excision of the P-element insertion EP3214 are depicted by interrupted lines. Genomic transgenes carrying wild-type (ube3a+) and mutant (ube3aC941A) containing a missense mutation in the codon encoding the catalytic cysteine residue ube3a are shown. (C) Western results of adult head extracts from various genotypes using the monoclonal antibody 8F7 against Ube3a. NOE denotes elav-Gal4/UAS-ube3a. No Ube3a expression was observed in hemizygous ube3a549 (ube3a549/Df(3L)ED4470) and ube3a689 (ube3a689/Df(3L) ED4470) mutants as well as homozygous ube3a35 mutants. A truncated Ube3a of ~110 kDa was observed in ube3a8 homozygous mutants. Tubulin was used as a loading control.