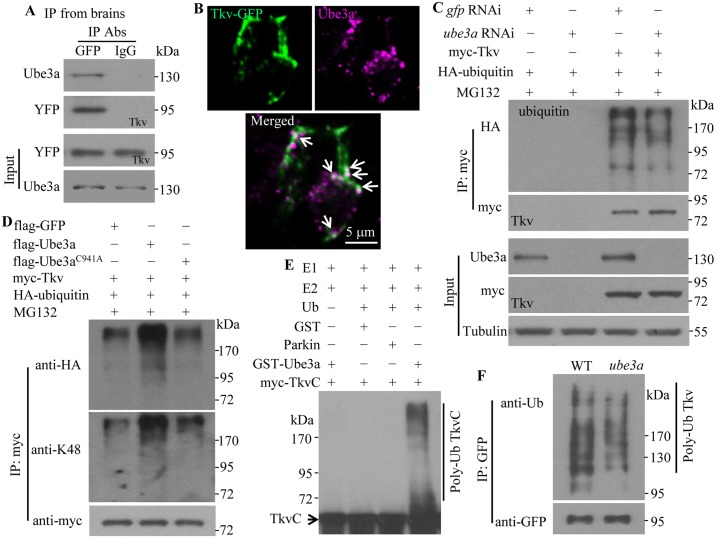
Fig 7. Ube3a interacts with and ubiquitinates Tkv in vivo and in vitro.
(A) Ube3a interacts with Tkv in larval brains as detected by co-IP. Ube3a was co-immunoprecipitated by anti-GFP from larval brain lysates of a gene trap line expressing endogenous Tkv tagged with YFP. (B) Co-localization of Tkv-GFP and Ube3a driven by elav-Gal4 in motoneuron soma. Images from two consecutive single slices are presented. Arrows indicate co-localized puncta. (C) Decreased Tkv ubiquitination by RNAi knockdown of Ube3a in S2 cells. Cells were co-transfected with plasmids encoding myc-tagged Tkv and HA-tagged ubiquitin, and treated with dsRNA against Ube3a or GFP dsRNA as a control. Anti-HA antibody was used to detect ubiquitinated Tkv. Tubulin was used as a loading control. (D) Increased Tkv poly-ubiquitination by overexpression of wild-type but not C941A mutant Ube3a in S2 cells. Poly-ubiquitination was detected using anti-HA and anti-K48 antibodies. (E) GST-Ube3a ubiquitinates myc-TkvC in vitro. The reaction was reconstituted with the components E1, E2, HA-Ub, the potential substrate myc-TkvC, together with the GST control, the E3 ligase Parkin control, or GST-Ube3a. Both poly-ubiquitinated and non-ubiquitinated TkvC are indicated. (F) In vivo ubiquitination of Tkv. Tkv-GFP from larval brain lysates was immunoprecipitated with anti-GFP and the level of ubiquitinated Tkv was detected by anti-ubiquitin. A decreased level of Tkv ubiquitination was observed in ube3a35 mutants compared with wild type.