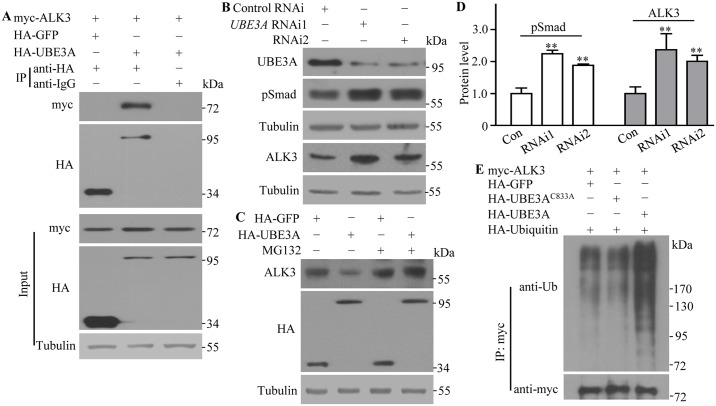
Fig 9. Human UBE3A negatively regulates the protein level of BMP receptor ALK3 in HEK293 cells.
(A) Human UBE3A interacts with the BMP receptor ALK3 in HEK293 cells. HEK293 cells were co-transfected with myc-tagged ALK3 and HA-tagged UBE3A or GFP as a control. Lysates from transfected cells were immunoprecipitated with anti-HA and anti-IgG as a control, followed by western blotting to detect the presence of HA- and myc-tagged proteins. (B) Increased levels of the endogenous pSmad protein and ALK3 were observed in HEK293 cells expressing reduced levels of UBE3A by two independent siRNAs. (C) A decreased level of the endogenous ALK3 protein was observed in UBE3A-overexpressing cells; this effect was abolished by treatment with the proteasome inhibitor MG132. (D) Statistical analysis of pMAD and ALK3 protein levels in HEK293 cells expressing reduced levels of UBE3A by two independent siRNAs (n = 4, one-way ANOVA test, mean ± SEM, **P < 0.01). (E) HA-tagged GFP, wild-type or C833A UBE3A was co-expressed with myc-ALK3 and HA-Ub in HEK293 cells as indicated. Lysates from transfected cells were immunoprecipitated with anti-myc and the levels of ubiquitination were analyzed with an anti-ubiquitin antibody.