abstract
Seminal root growth is one of the factors to determine rice seedling establishment. Our previous reports showed light can induce Z-type wavy root and coiling root morphology in several rice (Oryza sativa L.) varieties, and the regulated Z-type and unregulated coil seminal roots were resulted by different circumnutational trajectories. Moreover, the light-induced seminal root waving was conducted by an NO-dependent signaling pathway. In order to further reveal the difference of root tip movement between straight and wavy seminal roots; here, the root tip movement trajectories of Tainung 67 variety (TNG67; presented straight root in light conditions) and Taichung Native 1 (TCN1; presented Z-type wavy root in light) were recorded and analyzed in both white light and dark (dim far-red light was applied in dark for taking time-lapse photography) conditions. The results showed the root tip movement of both rice varieties in low intensity of dim far-red light conditions were followed the circumnutation path. However, the stimuli of high intensity of white light would increase the root helix angle in TCN1 seedlings but not in TNG67. In addition, slowing down the rate of root helix was induced by white light treatment in TCN1 but not in TNG67 seedlings. In conclusion, changes of TCN1 rice seminal root morphology from straight to wavy type stimulated by light was resulted by both helix angle increasing and circumnutation rate slowing of root tip movement.
Keywords: Circumnutation; oryza sativa, photomorphogenesis, seminal root, wavy root
Circumnutation is a spiral movement of growing organs in plants, and it was firstly described by Darwin in the book “The power of movement in plants.”1 Circumnutation was considered as an internal activity of plants. However, some reports had also indicated that the regularity of circumnutation pattern could be affected by external stimuli such as gravity, moisture, touch, and light.2,3
Light is not only the energy source for plant growth and development, but also an environmental signal to modulate plant photomorphogenesis for helping plants adapt to environmental conditions.4-7 Several mechanisms of photomorphogenesis in rice (Oryza sativa L.) such as inhibition of seminal root elongation and coleoptile growth have been studied.8-10 Our previous works showed light stimulate induced Taichung Native 1 (TCN1; an indica type rice) displayed a regular Z-type wavy phenotype in young seedlings under light conditions to replace a straight root phenotype in the dark. Tainung 67 (TNG67), a japonica rice variety, presented a straight root phenotype in both light and dark conditions.11 However, Taichung Sen 17 (an indica-type rice, TCS17), demonstrated irregular coiling seminal roots both in light and dark.11 Thus, it was suggested that genetics factor plays an important role to determine light sensitivities and effects on plant morphology and physiological responses. Based on the root tip movement recorded by time-lapse photography, it was already known that the difference of root phenotype in light conditions between TCN1 and TCS17 was resulted by various root tip movement trajectories.11 However, it is still unclear whether the straight and the Z-type wavy roots of TCN1 seedlings in dark and light conditions, respectively, were conducted by different movement trajectories of root tips or helix characteristics (e.g. helix angle and rate).
In this report, the movement of root tips of TCN1 and TNG67 seedlings in light and dark conditions were observed and analyzed. Since automatic-timed photography could not be carried out in the completely dark condition, the dim far-red light (D-FR, 3 μmol m−2 s−1) was applied for taking photography to record the root tip movement in the dark condition. Our previous experiment result also indicated wavy root morphology would not be presented if the white light intensity was down to 3 μmol m−2 s−1.11 The straight root morphology under 3 μmol m−2 s−1 of D-FR light conditions was the same with that in dark (Fig. 1A), and the results of root tip trajectory analysis showed that root tips of both rice varieties moved with circumnutational trajectories under both dim far-red light and white light conditions (100 μmol m−2s−1) (Fig. 1B). In TCN1 seedlings, the helix angle of root tip movement was about ±20 degrees under dim far-red light conditions, and the circumnutation rate was 1.1 cycle per hr. The record of the TCN1 seedling under white light conditions showed that the helix angle of root tip movement was also about ±20 degrees and the circumnutation rate was about 1.0 cycle per hr during the beginning 24 hr-light treatment. However, the angle of root tip movement was up more than ±40 degrees after 24-hr light exposed; in addition, the rotation rate was decreased to around 0.3 cycle per hr (Fig. 1B). The result of helix rate of TCN1 root tip in 100 μmol m−2 s−1 of white light condition showed here was consistent with our previous observation of the helix period (about 4.7 hr) of TCN1 root tip movement under a situation with 90 μmol m−2 s−1 of light.11 Comparison of the root tip behaviors of TCN1 seedlings in dim far-red light and white light suggested that alterations of helix angle and rate of root tip movement conducted the root morphology change from straight to visible wavy phenotype. On the other hand, the helix angle of root tip movements of TNG67 rice seedlings were about ±20 degrees under both dim far-red light and white light conditions, and the helix rate were about 0.8 and 0.7 cycle per hr, respectively. In other words, the angle of root tip movement and the period of circumnutation were not obviously changed by illumination in TNG67 seedlings (Fig. 1B). Comparison of the circumnutation patterns between TCN1 and TNG67 indicated light promoted root helix angle increasing and helix rate decreasing were variety-dependent. Moreover, the root tips movement of both straight root and wavy root during growth followed regular helix trajectory but the higher degree helix angle and slower helix rate conducted the visible Z-type wavy root morphology.
Figure 1.
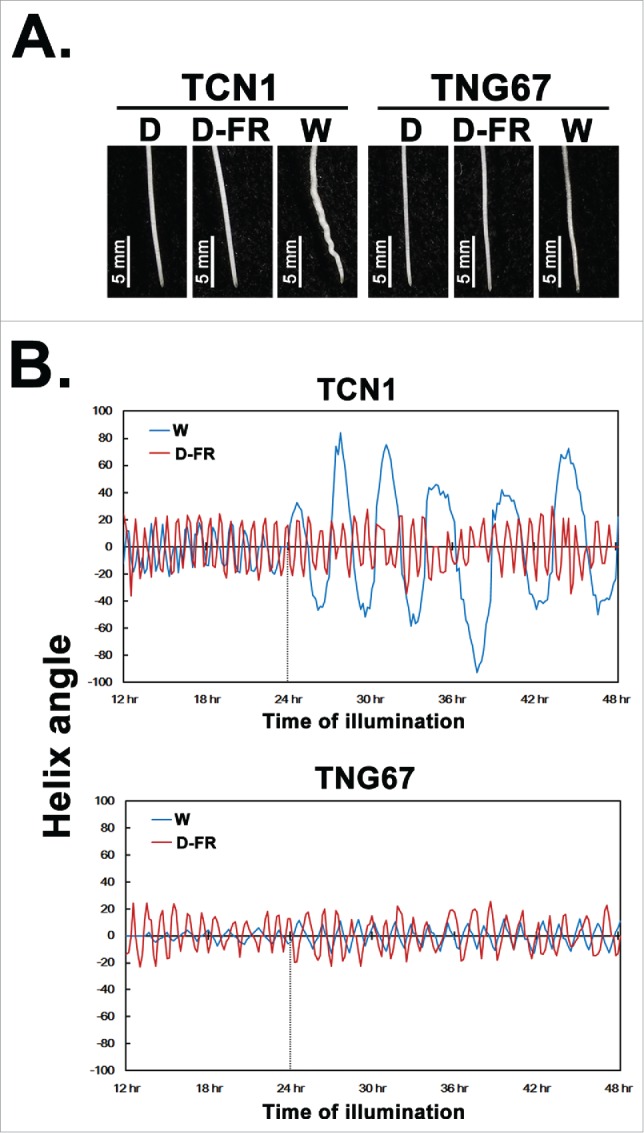
Light modulate root tip movement of rice young seedlings. The germinated rice (TCN1 and TNG67) seeds were grown in water. Seedlings were treated with white light, dim far-red light, and dark. Morphology of root tips (A), helix angles and rate of root tip movement (B) were recorded. W, 100 μmol m−2s−1 of white light; D, dark; D-FR, 3 μmol m−2 s−1 of dim far-red light.
Our previous studies indicated light stimuli triggers NO production in TCN1 rice seminal roots and induces wavy roots via the signals of oxylipins, ethylene and auxin in that order.12 Revealing whether the sensitivities to light stimuli or/and above mentioned cellular signals were different between TCN1 and TNG67 rice varieties could be expected to provide more information for understanding the interactions of genetic and environmental factors (i.e. light) in rice plants.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Agricultural Experimental Farm of National Taiwan University for providing the rice growing field for rice grains collection.
References
- 1.Darwin C. The power of movement in plants 1880. London: John Murray [Google Scholar]
- 2.Migliaccio F, Tassone P, Fortunati A. Circumnutation as an autonomous root movement in plants. Am J Bot 2013; 100:4-13; PMID:23243099; http://dx.doi.org/ 10.3732/ajb.1200314 [DOI] [PubMed] [Google Scholar]
- 3.Roy R, Bassham DC. Root growth movements: waving and skewing. Plant Sci 2014; 221-222:42-7; PMID:24656334; http://dx.doi.org/ 10.1016/j.plantsci.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 4.Quail PH, Boylan MT, Parks BM, Short TW, Xu Y, Wagner D. Phytochromes: photosensory perception and signal transduction. Science 1995; 268:675-80; PMID:7732376; http://dx.doi.org/ 10.1126/science.7732376 [DOI] [PubMed] [Google Scholar]
- 5.von Arnim A, Deng XW. Light control of seedling development. Annu Rev Plant Physiol Plant Mol Biol 1996; 47:215-43; PMID:15012288; http://dx.doi.org/ 10.1146/annurev.arplant.47.1.215 [DOI] [PubMed] [Google Scholar]
- 6.Kami C, Lorrain S, Hornitschek P, Fankhauser C. Light-regulated plant growth and development. Curr Top Dev Biol 2010; 91:29-66; PMID:20705178; http://dx.doi.org/ 10.1016/S0070-2153(10)91002-8 [DOI] [PubMed] [Google Scholar]
- 7.Casal JJ. Photoreceptor signaling networks in plant responses to shade. Annu Rev Plant Biol 2013; 64:403-27; PMID:23373700; http://dx.doi.org/ 10.1146/annurev-arplant-050312-120221 [DOI] [PubMed] [Google Scholar]
- 8.Takano M, Inagaki N, Xie X, Yuzurihara N, Hihara F, Ishizuka T, Yano M, Nishimura M, Miyao A, Hirochika H, Shinomura T. Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice. Plant Cell 2005; 17:3311-25; PMID:16278346; http://dx.doi.org/ 10.1105/tpc.105.035899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie X, Shinomura T, Inagaki N, Kiyota S, Takano M. Phytochrome-mediated inhibition of coleoptile growth in rice: age-dependency and action spectra. Photochem Photobiol 2007; 83:131-8; PMID:17029495; http://dx.doi.org/ 10.1562/2006-03-17-RA-850 [DOI] [PubMed] [Google Scholar]
- 10.Shimizu H, Tanabata T, Xie X, Inagaki N, Takano M, Shinomura T, Yamamoto KT. Phytochrome-mediated growth inhibition of seminal roots in rice seedlings. Physiol Plant 2009; 137:289-97; PMID:19744160; http://dx.doi.org/ 10.1111/j.1399-3054.2009.01277.x [DOI] [PubMed] [Google Scholar]
- 11.Wang SJ, Ho CH, Chen HW. Rice develops wavy seminal roots in response to light stimulus. Plant Cell Rep 2011; 30:1747-58; PMID:21573806; http://dx.doi.org/ 10.1007/s00299-011-1082-2 [DOI] [PubMed] [Google Scholar]
- 12.Chen HW, Shao KH, Wang SJ. Light-modulated seminal wavy roots in rice mediated by nitric oxide-dependent signaling. Protoplasma 2015; 252:1291-304; PMID:25619895; http://dx.doi.org/ 10.1007/s00709-015-0762-0 [DOI] [PubMed] [Google Scholar]


