Figure 1.
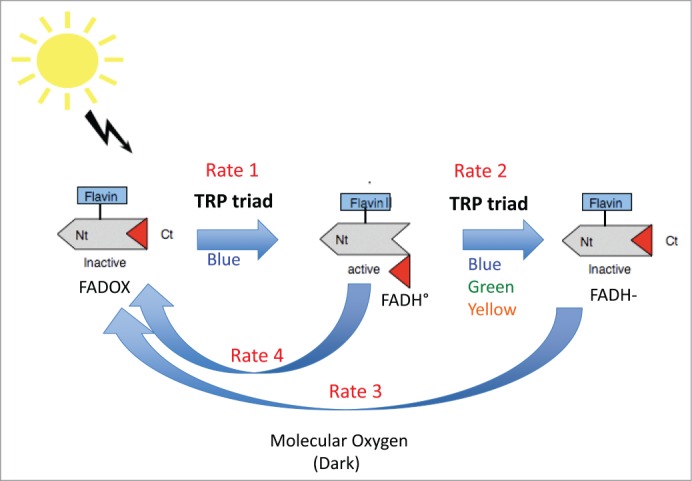
Presumed photocycle of plant cryptochromes. In the dark, cryptochromes are in the inactive state (C-terminal domain folded against the protein, flavin in the oxidized redox state). Upon illumination, flavin undergoes photoreduction to the FADH° redox form, which triggers conformational unfolding of the C-terminal domain to give the activated form of the receptor. Subsequent illumination of FADH° reduces the flavin further to the inactive (FADH-) redox form. Both radical (FADH°) and fully reduced (FADH-) flavin are reoxidized to the inactive (FAD) form by molecular oxygen.10 The receptor undergoes continuous cycling between redox forms under illumination and the degree of activation depends on the equilibrium reached (rates 1, 2, 3, 4) between reducing and oxidizing reactions. In this mechanism, the Trp triad mediates forward electron transfer driving forward redox state interconversion.
