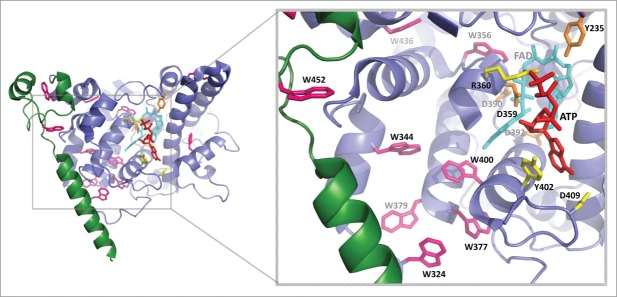
Figure 5.
Electron transfer pathways and possible C-terminal domain structure in A. thaliana cryptochrome 1. Structure was taken from PDB: 1U3D. Tryptophan residues are shown in pink, including positions of the classical Trp triad (FAD -W400 - W377 - W324) and residues implicated in alternate electron transfer pathways deduced from cry2 including aromatic amino acid residues near the Trp triad (cry1 W334, W379). The cry1 Y402 residue (homolog to cry2 Y399) is suitably positioned to form a potential electron tunneling pathway via ATP.14 The FAD is shown in cyan and the FAD binding amino acids, which may impact on structural changes in the protein are in orange. The ATP analog AMPPNP is in red. The fragment of C-T terminal analyzed in this study is in green and is lacking in the crystal structure. Created in the PyMOL Molecular Graphics System, Version 1.7.6.3 Schrödinger, LLC. Created in PyMOL.