FIGURE 6:
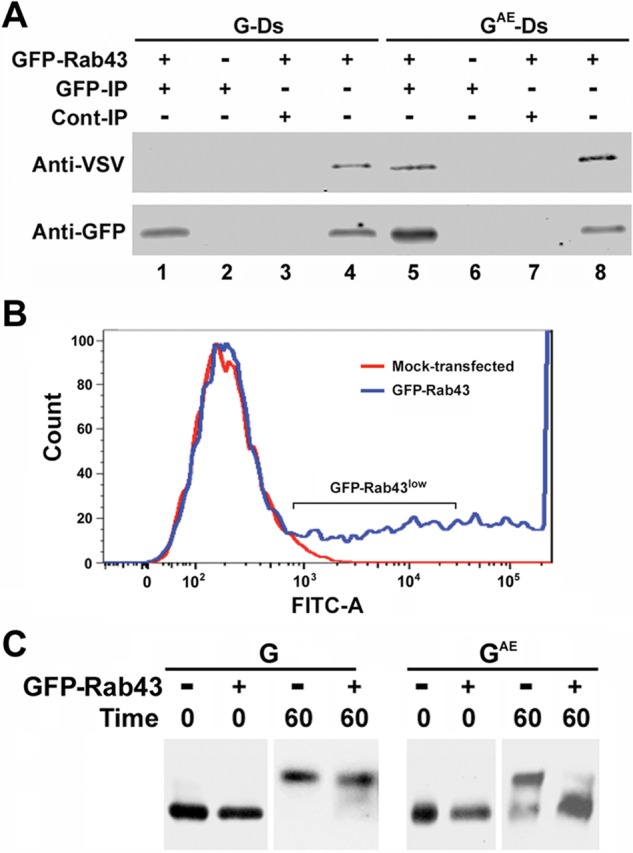
Coprecipitation of GAE with GFP-Rab43. (A) COS7 cells expressing GFP-Rab43 were infected with adenovirus encoding GAE-Ds or G-Ds and grown at restrictive temperature for 24 h. The cells were then shifted to permissive temperature for 60 min and harvested. After being washed in PBS, the cells were lysed by sonication and centrifuged at 5000 × g for 10 min. The supernatants were then subjected to immunoprecipitation analysis using rabbit anti-GFP (GFP) antibodies (lanes 1 and 5), and the precipitates were immunoblotted with an anti-VSV antibody that recognizes the identical ectodomains of GAE and G and anti-GFP antibodies. Control GFP immunoprecipitates were prepared from cells that did not express GFP-Rab43 (lanes 2 and 6). Additional control immunoprecipitates were prepared using protein A agarose beads coated with normal rabbit serum (lanes 3 and 7). Lysates are included for comparison (lanes 4 and 8). (B) COS7 cells were transfected with GFP-Rab43, and 24 h posttransfection, cells expressing low levels of GFP-Rab43 were isolated using a fluorescence-activated cell sorter. (C) The GFP-Rab43low cells or unsorted mock-transfected cells were replated and infected with adenovirus encoding GAE or G. The cells were grown overnight at restrictive temperature and lysed (0 time) or shifted to permissive temperature for 60 min before lysis. In each instance, the lysates were incubated with endoglycosidase H and subjected to immunoblotting analysis with the anti-VSV antibody, which recognizes the identical ectodomains of GAE and G. GFP-Rab43 expression significantly blocked the acquisition of complex N-linked sugars by GAE but had minimal effect on the acquisition of complex N-linked sugars by G.
