Abstract
Several studies suggest a link between post-transplant hypomagnesemia and new-onset diabetes after transplantation (NODAT), but this relationship remains controversial. We conducted a retrospective cohort study of 948 nondiabetic kidney transplant recipients from January 1, 2000, to December 31, 2011, to examine the association between serum magnesium level and NODAT. Multivariable Cox proportional hazards models were fitted to evaluate the risk of NODAT as a function of baseline (at 1 month), time-varying (every 3 months), and rolling-average (i.e., mean for 3 months moving at 3-month intervals) serum magnesium levels while adjusting for potential confounders. A total of 182 NODAT events were observed over 2951.2 person-years of follow-up. Multivariable models showed an inverse relationship between baseline serum magnesium level and NODAT (hazard ratio [HR], 1.24 per 0.1 mmol/L decrease; 95% confidence interval [95% CI], 1.05 to 1.46; P=0.01). The association with the risk of NODAT persisted in conventional time-varying (HR, 1.32; 95% CI, 1.14 to 1.52; P<0.001) and rolling-average models (HR, 1.34; 95% CI, 1.13 to 1.57; P=0.001). Hypomagnesemia (serum magnesium <0.74 mmol/L) also significantly associated with increased risk of NODAT in baseline (HR, 1.58; 95% CI, 1.07 to 2.34; P=0.02), time-varying (HR, 1.78; 95% CI, 1.29 to 2.45; P<0.001), and rolling-average models (HR, 1.83; 95% CI, 1.30 to 2.57; P=0.001). Our results suggest that lower post-transplant serum magnesium level is an independent risk factor for NODAT in kidney transplant recipients. Interventions targeting serum magnesium to reduce the risk of NODAT should be evaluated.
Keywords: kidney transplantation, diabetes mellitus, hypomagnesemia
The burden of new-onset diabetes after transplantation (NODAT) has become increasingly clear since the late 1990s.1–4 In a 10-year cohort study, 24% of kidney transplant recipients (KTR) developed NODAT within 3 years of transplantation, many of whom were diagnosed in the early post-transplant period.4 Compared with nondiabetic KTR, patients with NODAT or pretransplant diabetes experienced worse long-term allograft function and higher risks of graft failure, post-transplant cardiovascular disease, and mortality.1,4–9
Various risk factors for the development of NODAT have been identified, including age, ethnicity, obesity, family history of diabetes, acute rejection, cytomegalovirus infection, hepatitis C, use of corticosteroids, and notably, magnesium (Mg) deficiency.1–4,10–14 Mg is an intracellular cofactor necessary for glucose transport between membranes, glucose oxidation, and insulin-mediated tyrosine kinase pathways.15,16 Hypomagnesemia (hypoMg) is commonly observed among KTR who are prescribed calcineurin inhibitors (CNI). It has been suggested that CNI downregulate Mg transport proteins in the renal tubules, leading to increased Mg excretion and wasting.17–20
Several studies in nontransplant populations have shown that higher consumption of Mg is associated with a lower risk of diabetes mellitus.21–26 Other studies have reported a higher incidence of hypoMg among patients with diabetes or prediabetic hyperglycemia compared with nondiabetic controls.27–30 Randomized control trials have demonstrated that type II diabetic patients who receive Mg supplement exhibit improved glycemic control.31,32 In the context of kidney transplantation, however, this association remains controversial due to conflicting findings.33–37 Moreover, previous studies have methodologic limitations such as not properly accounting for time-varying serum Mg levels over follow-up.
In view of these limitations, we aimed to examine the association between serum Mg and risk of NODAT in a large cohort of KTR using comprehensive data from a single-center research database. To appropriately account for exposure over follow-up, serum Mg was modeled as a time-fixed, conventional time-varying, and rolling time-varying average exposure in multivariable analyses to determine its role in the development of NODAT.
Results
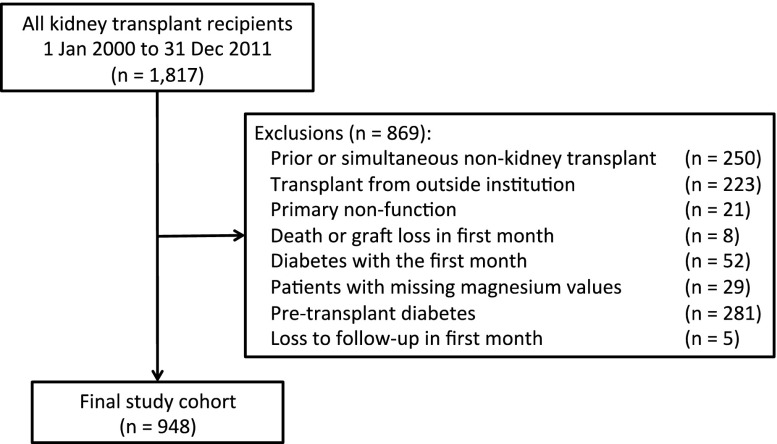
We identified 1817 KTR who were transplanted between January 1, 2000 and December 31, 2011 in the Comprehensive Renal Transplant Research and Information System database. The prevalence of pretransplant diabetes mellitus was 29% and remained relatively stable throughout the study period (Supplemental Appendix 1). Figure 1 depicts the patients excluded from the study cohort after application of the exclusion criteria. The final study cohort consisted of 948 KTR (Figure 1).
Figure 1.
Study flow diagram showing the results of applying exclusion criteria to the cohort of patients eligible for study inclusion.
A total of 182 NODAT events were documented during 2951.2 person-years at risk. The median follow-up was 3.4 years (95% confidence interval [95% CI], 1.4 to 5.0). The median time to NODAT was 0.6 years (95% CI, 0.1 to 2.1). The incidence rate of NODAT was 6.2 (95% CI, 5.3 to 7.1) per 100 person-years. Recipient, donor, and transplant baseline characteristics are presented in Table 1. The mean recipient age was 47.4 years (±13.3). There was a preponderance of males (60.7%) and patients of White race (70.2%) in the study cohort. The median time on dialysis prior to transplant was 3.2 years (95% CI, 1.0 to 5.9). The prevalence of hypoMg at 1 month post-transplant was 38.6%. Slightly over half of the patients received kidneys from living donors. At 1 month post-transplant, 64.5% and 35.5% of KTR were receiving tacrolimus and cyclosporine, respectively.
Table 1.
Baseline characteristics of the study cohort
| Baseline Study Characteristics | Number of Patients (n=948) | Measurements |
|---|---|---|
| Recipient age (years±SD) | 948 | 47.4 (±13.3) |
| Recipient sex | ||
| Male | 575 | 60.7% |
| Female | 373 | 39.4% |
| Recipient race | ||
| Non-White | 280 | 29.9% |
| White | 658 | 70.2% |
| Median recipient BMI (kg/m2, interquartile range) | 846 | 24.8 (21.7, 28.4) |
| Peak PRA (%) | ||
| =0% | 453 | 48.8% |
| >0% | 475 | 51.2% |
| Median time on dialysis (years, interquartile range) | 943 | 3.2 (1.0, 5.9) |
| Mean recipient eGFR (CKD-EPI) at one month (ml/min per 1.73 m2, ±SD) | 931 | 56.9 (±20.7) |
| Hypomagnesemia at 1 month post-transplant | ||
| No | 582 | 61.4% |
| Yes | 366 | 38.6% |
| Cause of ESRD | ||
| Nonglomerulonephritis | 533 | 56.2% |
| Glomerulonephritis | 415 | 43.8% |
| Donor age | 929 | 44.5 (±13.1) |
| Donor sex | ||
| Male | 470 | 50.5% |
| Female | 461 | 49.5% |
| Mean donor BMI (kg/m2, ±SD) | 898 | 26.5 (±5.3) |
| Donor type | ||
| Deceased | 456 | 48.1% |
| Living | 492 | 51.9% |
| Donor history of hypertension | ||
| No | 743 | 86.1% |
| Yes | 120 | 13.9% |
| Delay graft function | ||
| No | 776 | 81.9% |
| Yes | 172 | 18.1% |
| Types of CNI at one month | ||
| Tacrolimus | 586 | 64.5% |
| Cyclosporine | 323 | 35.5% |
| Regraft | ||
| No | 808 | 85.2% |
| Yes | 140 | 14.8% |
| Transplant era | ||
| 2000–2004 | 323 | 34.1% |
| 2005–2008 | 326 | 34.4% |
| 2009–2011 | 299 | 31.5% |
BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; PRA, panel reactive antibody.
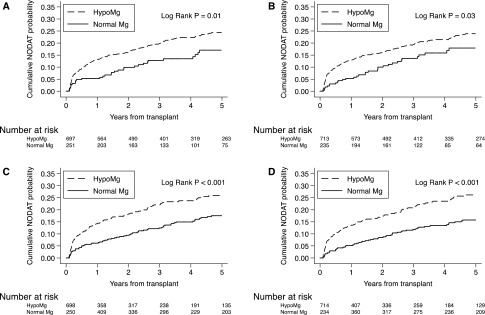
Figure 2 illustrates Kaplan–Meier curves showing the cumulative probability of NODAT stratified by serum Mg categories (i.e., hypoMg versus normal Mg). When using baseline serum Mg level at 1 month post-transplant, the hypoMg group displayed a higher cumulative probability of NODAT than the normal Mg group throughout the follow-up period (24.3% versus 17.1% at 5 years, P=0.01; Figure 2A). When using mean serum Mg over the first month after transplant, the cumulative probability of NODAT in the hypoMg group was significantly higher than the normal Mg group (23.9% versus 17.9% at 5 years, P=0.03; Figure 2B). Treating serum Mg level as a time-varying exposure showed that KTR with hypoMg experienced a higher cumulative probability of NODAT compared with KTR with normal Mg levels (26.0% versus 17.5% at 5 years, P<0.001; Figure 2C). Finally, when hypoMg was considered a rolling-average exposure, based on the mean serum Mg level over the prior 3 months, the curves showed a separation early post-transplant and the difference in the probability of developing NODAT continued to increase over time (26.2% versus 15.8% at 5 years, P<0.001; Figure 2D).
Figure 2.
Kaplan–Meier curves for NODAT using different approaches to analyze Mg levels post-transplant. Cumulative probabilities of NODAT by (A) serum Mg at 1-month post-transplant; (B) average serum Mg over the first month post-transplant; (C) time-varying serum Mg updated at 3-month intervals; and (D) rolling-average time-varying serum Mg assessed over 3-month exposure windows.
The results of the time-fixed multivariable Cox proportional hazards models are displayed in Table 2. When analyzed as a continuous variable, a lower baseline serum Mg at 1 month post-transplant was associated with an increased risk of NODAT (hazard ratio [HR], 1.24 per 0.1 mmol/L decrease in Mg; 95% CI, 1.05 to 1.46; P=0.01). The inverse relationship between serum Mg and risk of NODAT persisted when using mean serum Mg measured over the first month post-transplant (HR, 1.21; 95% CI, 1.02 to 1.45; P=0.03). Similarly, when serum Mg was treated as a binary variable, KTR with hypoMg at baseline had a 58% increase in the risk for developing NODAT compared with KTR without hypoMg (HR, 1.58; 95% CI, 1.07 to 2.34; P=0.02). However, hypoMg did not reach statistical significance when using mean serum Mg measured over the first month post-transplant (HR, 1.40; 95% CI, 0.94 to 2.07; P=0.10).
Table 2.
Cox proportional hazards model for the risk of NODAT by time-fixed serum Mg levels
| Exposure Variables | Time-Fixed Magnesium Level | ||
|---|---|---|---|
| HR (95% CI) | P Value | ||
| Continuous Mg | Mg (per 0.1 mmol/L decrease) | 1.24 (1.05 to 1.46) | 0.01 |
| Average Mg in the first month post-transplant | 1.21 (1.02 to 1.45) | 0.03 | |
| Dichotomous Mg | Hypomagnesemia (Yes versus No) | 1.58 (1.07 to 2.34) | 0.02 |
| Average Mg in the first month post-transplant | 1.40 (0.94 to 2.07) | 0.10 | |
Unit conversion for serum Mg: 1 mmol/L=2.43 mg/dl. Models were adjusted for recipient age, sex, race, body mass index, peak PRA, time on dialysis, eGFR, pretransplant hypomagnesemia, cause of ESRD, donor age, sex, history of hypertension, body mass index, donor type, delayed graft function, regraft, type of CNI at baseline, and transplant era. PRA, panel reactive antibody.
The results of the time-dependent multivariable Cox proportional hazards models are presented in Table 3. When using a conventional time-varying model to assess changes in serum Mg levels every 3 months, a lower serum Mg was associated with an increased risk of NODAT (HR, 1.32 per 0.1 mmol/L decrease in Mg; 95% CI, 1.14 to 1.52; P<0.001). Additionally, hypoMg was significantly associated with an increased risk of NODAT compared with the normal Mg group (HR, 1.78; 95% CI, 1.29 to 2.45; P<0.001). Similar to the conventional time-varying model, the 3-month rolling-average model showed a significant inverse relationship between serum Mg level and risk of NODAT (HR, 1.34 per 0.1 mmol/L decrease in serum Mg; 95% CI, 1.13 to 1.57; P=0.001). Moreover, KTR with hypoMg experienced an 83% increased risk for developing NODAT compared with KTR without hypoMg in the rolling-average model (HR, 1.83; 95% CI, 1.30 to 2.57; P=0.001).
Table 3.
Cox proportional hazards model for the risk of NODAT by time-varying serum Mg levels
| Exposure Variables | Time-Varying Magnesium Level | ||
|---|---|---|---|
| HR (95% CI) | P Value | ||
| Continuous Mg | Mg (per 0.1 mmol/L decrease) | 1.32 (1.14 to 1.52) | <0.001 |
| Rolling average of Mg in the previous 3 months | 1.34 (1.13 to 1.57) | 0.001 | |
| Dichotomous Mg | Hypomagnesemia (Yes versus No) | 1.78 (1.29 to 2.45) | <0.001 |
| Rolling average of Mg in the previous 3 months | 1.83 (1.30 to 2.57) | 0.001 | |
Unit conversion for serum Mg: 1 mmol/L=2.43 mg/dl. Models were adjusted for recipient age, sex, race, body mass index, peak PRA, time on dialysis, eGFR, pretransplant hypomagnesemia, cause of ESRD, donor age, sex, history of hypertension, body mass index, donor type, delayed graft function, regraft, type of CNI at baseline, and transplant era.
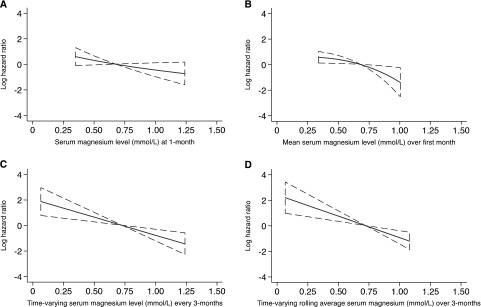
The continuous relationship between serum Mg exposure and the log HR for the risk of NODAT was demonstrated using the method of fractional polynomials (Figure 3). Consistent with the results of the multivariable analyses, serum Mg levels were inversely associated with the risk of NODAT in a log-linear fashion across the range of serum Mg studied.
Figure 3.
Fractional polynomial plots for the risk of NODAT using different approaches to analyze Mg levels post-transplant. Log hazard ratios for NODAT by (A) serum Mg at 1-month post-transplant; (B) mean serum Mg over the first month post-transplant; (C) time-varying serum Mg updated at 3-month intervals; and (D) rolling-average time-varying serum Mg assessed over 3-month exposure windows.
Additional analyses showed that the exposure-outcome association was not significantly modified by various patient subgroups (Supplemental Appendix 2). Moreover, Cox models sequentially fitted with an expanding set of covariates showed essentially no change in the main association (Supplemental Appendix 3). Sensitivity analyses that excluded patients with early hyperglycemia diagnosed NODAT from glucose readings alone or the initiation of diabetes treatment, and adjusted for exposure to supratherapeutic CNI levels in the first month post-transplant showed similar results to the main analysis (Supplemental Appendices 4–6). Pretransplant Mg exhibited no correlation with 1-month post-transplant Mg (Supplemental Appendix 7) and an absence of an association was confirmed in both time-fixed and time-dependent Cox models (Supplemental Appendix 8).
Discussion
Using a large, single-center cohort of KTR, we aimed to comprehensively assess post-transplant hypoMg as an independent risk factor for the development of NODAT. Similar to the results of the Kaplan–Meier analyses, the time-fixed Cox proportional hazard model indicated that a lower serum Mg was significantly associated with an increased risk of NODAT after adjustment for recipient, donor, and transplant factors. Using a conventional time-varying Cox model also showed that a lower serum Mg measured every 3 months was associated with a greater risk of NODAT. Accounting for changes in serum Mg levels using a rolling-average model further demonstrated this inverse relationship between serum Mg level and risk of NODAT.
Several past cohort studies have examined the relationship between serum Mg and NODAT, but they have shown conflicting results, likely due to differences in how serum Mg levels were analyzed. Osorio et al. investigated this relationship among 589 nondiabetic KTR whose serum Mg measurements were performed throughout the first year post-transplant.34 They reported no association between hypoMg and NODAT after adjustment for CNI regimen. In contrast, Van Laecke et al. conducted a study of 390 KTR to assess the relation between hypoMg and NODAT, using serum Mg levels during the first 2 months after transplant.36 They found that although the use of CNI was associated with an increased likelihood of developing NODAT, hypoMg at 1 month post-transplant was also an independent risk factor. The same research group published similar results based on a cohort of 169 liver transplant recipients.35 The findings of Van Laecke et al. were later supported by a study on KTR in the United States.35
More recently, Van Laecke et al. conducted a randomized control trial to examine the effectiveness of Mg supplementation to improve glycemic control and insulin sensitivity after kidney transplantation.38 They reported that KTR on Mg supplementation displayed lower fasting glycemia at 3 months post-transplant compared with controls. However, no significant differences were noted in other measures of glycemia. The trial was not powered to evaluate the effect of Mg supplementation on the risk of NODAT over follow-up.
In order to address the limitations of prior time-fixed analyses, we incorporated conventional time-varying and rolling-average Cox proportional hazards models. The principal finding of the study is that hypoMg is a strong independent risk factor for NODAT. Compared with the results of time-fixed models, the conventional and rolling-average models showed markedly higher hazard ratios. This suggests that serum Mg may impose a stronger risk for developing NODAT when it is properly estimated as a time-dependent exposure.
The pathophysiologic relationship between hypoMg and NODAT is complex. A comprehensive review suggested that hypoMg is associated with altered cellular transport of glucose, reduced insulin secretion by β-cells within the pancreas, and defective insulin signaling pathways.39,40 On the contrary, type II diabetes mellitus may induce hypoMg due to gastrointestinal (e.g., gastroparesis and diarrhea), renal (e.g., reduced tubular reabsorption due to impaired insulin-mediated Mg transport), and other factors (e.g., diuretics, antibiotics, and antifungal agents).40–43 Our results (particularly the time-varying analyses) support the former view that hypoMg is an independent risk factor for NODAT.
This study used a large, well characterized, single-center cohort of KTR to evaluate the association of serum Mg levels and NODAT. Moreover, the study cohort’s comprehensive and repeated measures of serum Mg, along with other clinical/laboratory parameters, allowed for a detailed analysis of the study question. Finally, the study patients were transplanted in the contemporary era of immunosuppression and other clinical practice patterns remained relatively stable over the follow-up period.
Despite these strengths, the study has some limitations that deserve note. First, because Mg is mostly an intracellular cation, it has been questioned whether hypoMg can be accurately diagnosed with extracellular serum Mg.39 However, from a clinical perspective, it has been suggested that in patients with suspected Mg deficiency, a low serum Mg level is sufficient to confirm the diagnosis.15,16 Second, we did not incorporate Mg intake into the multivariable analyses. It has been shown that Mg supplementation is associated with a reduced risk of type II diabetes mellitus.31,32 Nevertheless, the intake of Mg should be reflected in the serum Mg level, which was measured and analyzed continuously throughout the course of follow-up. Third, missing data were minimal for the primary exposure and outcomes of interest, but some covariates contained incomplete information. We used the method of multiple imputation to appropriately account for missing covariate data with the assumption that the missingness was at random. Finally, residual confounding is often a concern when analyzing data from observational studies. We used a priori exclusion criteria and multivariable modeling with adjustment for potential confounders to reduce the impact of this source of bias.
In summary, our results suggest that a lower serum Mg level is associated with a quantitatively increased risk of NODAT, based on time-fixed, conventional time-varying, and rolling-average time-varying Cox proportional hazards models. The significant burden of hypoMg and its association with NODAT in the kidney transplant population warrant further research into interventions to prevent or correct hypoMg to reduce the risk of NODAT in KTR.
Concise Methods
Study Population
A retrospective cohort study was conducted on all patients ≥18 years of age who received transplants from January 1, 2000–December 31, 2011 (with follow-up until June 30, 2012) at the Toronto General Hospital, University Health Network. Exclusion criteria included: (1) prior or simultaneous nonkidney transplant, (2) kidney transplants from outside institutions, (3) primary nonfunction, (4) absence of serum Mg measurements post-transplant, (5) history of pretransplant diabetes mellitus, (6) NODAT within the first month post-transplant, and (7) graft failure, death, or loss to follow-up within the first month post-transplant. Immunosuppression protocols and patient follow-up schedules are described in Supplemental Appendix 9. Data sources are highlighted in Supplemental Appendix 10.44
Exposure Assessment and Classification
The exposure of interest was post-transplant serum Mg, which was analyzed as a continuous and categorical (binary) variable. HypoMg was defined as serum Mg level <0.74 mmol/L. Moreover, serum Mg was assessed as a time-fixed, conventional time-varying, and rolling-average time-varying exposure in the multivariable Cox proportional hazards models. Serum Mg at 1 month post-transplant was used for the time-fixed models. Conventional time-varying models updated the level of serum Mg at regular intervals (i.e., every 3 months) over follow-up and related this level to the risk of NODAT over the subsequent 3 months. The rolling-average time-varying model took the mean serum Mg calculated over a 3-month exposure window and attributed this value to the risk of NODAT over the subsequent 3-month risk window. The 3-month exposure window was then moved forward in time by 3-month increments to recalculate the mean serum Mg and assess its association with NODAT in the following risk window. In contrast to a conventional time-varying model, the rolling-average time-varying model more accurately reflects the average exposure to serum Mg during the 3-month exposure window prior to the period at risk for NODAT. A similar approach to exposure assessment and classification has been implemented by others.45
Outcome Assessment and Classification
The outcome of interest, NODAT, was defined as the occurrence of one or more of the following starting 1 month after kidney transplantation: (1) at least two fasting glucose readings ≥7.0 mmol/L (126 mg/dl), (2) at least two nonfasting glucose readings ≥11.1 mmol/L (200 mg/dl), or (3) the need for antidiabetic medications (including insulin) persisting beyond the first month after transplantation. Graft loss (i.e., return to chronic dialysis or preemptive retransplantation), death with graft function, or loss to follow-up prior to the development of NODAT were treated as censoring events in the survival analysis.
Potential Confounders
The following potential confounders were included in the multivariable Cox proportional hazards models: (1) recipient factors (age, sex, race, body mass index, peak panel reactive antibody level, time on dialysis, eGFR using the Chronic Kidney Disease Epidemiology Collaboration formula,46 and pretransplant hypoMg measured just prior to transplantation); (2) donor factors (age, sex, body mass index, deceased versus living donor transplant, and history of hypertension); and (3) transplant factors (regraft status, delayed graft function, type of CNI, proportion of CNI levels over therapeutic threshold [tacrolimus trough >15 μg/L, cyclosporine trough >250 μg/L, 2-hour cyclosporine >1700 μg/L] and transplant era). The following biochemic variables were also collected: pretransplant serum Mg, post-transplant serum Mg, glucose, and creatinine. These laboratory data were usually collected as part of the routine blood work performed at regular intervals in the post-transplant period.
Additional Analyses
The relation between serum Mg and the risk of NODAT was examined in the following patient subgroups: (1) median recipient age (<48 versus ≥48 years), (2) recipient sex (male versus female), (3) recipient race (White, Black, or Other), (4) recipient body mass index (<25 versus ≥25 kg/m2), and (5) CNI type (tacrolimus versus cyclosporine). In addition, the following sensitivity analyses were performed: (1) exclusion of patients with any hyperglycemia (i.e., fasting glucose ≥7.0 mmol/L [126 mg/dl] or nonfasting glucose ≥11.1 mmol/L [200 mg/dl]) within the first month post-transplant; (2) limiting the diagnosis of NODAT to glucose readings alone; (3) limiting the diagnosis of NODAT to patients initiating diabetes treatment alone (including insulin and/or oral agents); (4) adjustment for exposure to supratherapeutic CNI levels in the first month post-transplant; and (5) modeling pretransplant Mg on the risk of NODAT.
Statistical Analyses
Continuous baseline variables were presented as means±SD or medians (interquartile range) as appropriate. Categorical variables were analyzed with the chi-squared test. The time origin for the survival analyses was set to 1 month post-transplant. This allowed for the exclusion of very early NODAT events (which may have reflected undiagnosed diabetes pretransplant) and ensured that kidney function had stabilized prior to assessing Mg levels. The Kaplan–Meier method was used to examine the cumulative incidence of NODAT over time. The log rank statistic was used to assess differences across survival functions.
We fitted multivariable Cox proportional hazards models to determine the independent association of serum Mg level and the risk of NODAT for time-fixed, conventional time-varying, and rolling-average time-varying parameterizations of serum Mg. Violations in the proportional hazards assumption were assessed using Schöenfeld residuals and log (cumulative hazard) plots. No important departures were identified. To examine the continuous, and potentially nonlinear, relationship between serum Mg and NODAT, we used the fractional polynomial method to graphically depict the log HR of NODAT over a range of serum Mg values observed in the study cohort.47 The method of multiple imputation was applied to missing covariate data.
A two-sided P value of <0.05 was considered statistically significant. All analyses were performed using Stata/MP 12.1 (StataCorp, College Station, TX). The study was approved by the University Health Network Research Ethics Board. This report is presented in compliance with the Strengthening the Reporting of Observational studies in Epidemiology recommendations for observational studies.48
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Elizabeth Murakami for her excellent administrative support and critical reading of an earlier version of this manuscript. The authors also thank the students of the Multi-Organ Transplant Student Research Training Program for collecting, entering, and auditing data for the Comprehensive Renal Transplant Research and Information System at the Toronto General Hospital, University Health Network. No financial support was received to conduct this study.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015040391/-/DCSupplemental.
References
- 1.Bodziak KA, Hricik DE: New-onset diabetes mellitus after solid organ transplantation. Transpl Int 22: 519–530, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Davidson JA, Wilkinson A International Expert Panel on New-Onset Diabetes after Transplantation : New-Onset Diabetes After Transplantation 2003 International Consensus Guidelines: an endocrinologist’s view. Diabetes Care 27: 805–812, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Kaposztas Z, Gyurus E, Kahan BD: New-onset diabetes after renal transplantation: diagnosis, incidence, risk factors, impact on outcomes, and novel implications. Transplant Proc 43: 1375–1394, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ: Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 3: 178–185, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, Ferguson RM: Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int 62: 1440–1446, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Ducloux D, Kazory A, Chalopin JM: Posttransplant diabetes mellitus and atherosclerotic events in renal transplant recipients: a prospective study. Transplantation 79: 438–443, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Miles AM, Sumrani N, Horowitz R, Homel P, Maursky V, Markell MS, Distant DA, Hong JH, Sommer BG, Friedman EA: Diabetes mellitus after renal transplantation: as deleterious as non-transplant-associated diabetes? Transplantation 65: 380–384, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Montori VM, Basu A, Erwin PJ, Velosa JA, Gabriel SE, Kudva YC: Posttransplantation diabetes: a systematic review of the literature. Diabetes Care 25: 583–592, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Revanur VK, Jardine AG, Kingsmore DB, Jaques BC, Hamilton DH, Jindal RM: Influence of diabetes mellitus on patient and graft survival in recipients of kidney transplantation. Clin Transplant 15: 89–94, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Eckhard M, Martin I, Eich T, Weimer R, Zinn S, Bretzel RG, Brendel MD: Incidence of cytomegalovirus infections after immunosuppression induction in clinical islet transplantation and impact on graft function. Transplant Proc 34: 1922–1924, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Guerrero-Romero F, Rodríguez-Morán M: Hypomagnesemia, oxidative stress, inflammation, and metabolic syndrome. Diabetes Metab Res Rev 22: 471–476, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Hjelmesaeth J, Jenssen T, Hagen M, Egeland T, Hartmann A: Determinants of insulin secretion after renal transplantation. Metabolism 52: 573–578, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Hjelmesaeth J, Sagedal S, Hartmann A, Rollag H, Egeland T, Hagen M, Nordal KP, Jenssen T: Asymptomatic cytomegalovirus infection is associated with increased risk of new-onset diabetes mellitus and impaired insulin release after renal transplantation. Diabetologia 47: 1550–1556, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Leung Ki EL, Venetz JP, Meylan P, Lamoth F, Ruiz J, Pascual M: Cytomegalovirus infection and new-onset post-transplant diabetes mellitus. Clin Transplant 22: 245–249, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Tosiello L: Hypomagnesemia and diabetes mellitus. A review of clinical implications. Arch Intern Med 156: 1143–1148, 1996 [PubMed] [Google Scholar]
- 16.White JR Jr, Campbell RK: Magnesium and diabetes: a review. Ann Pharmacother 27: 775–780, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Markell MS, Altura BT, Sarn Y, Barbour R, Friedman EA, Altura BM: Relationship of ionized magnesium and cyclosporine level in renal transplant recipients. Ann N Y Acad Sci 696: 408–411, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Mazzola BL, Vannini SD, Truttmann AC, von Vigier RO, Wermuth B, Ferrari P, Bianchetti MG: Long-term calcineurin inhibition and magnesium balance after renal transplantation. Transpl Int 16: 76–81, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Navaneethan SD, Sankarasubbaiyan S, Gross MD, Jeevanantham V, Monk RD: Tacrolimus-associated hypomagnesemia in renal transplant recipients. Transplant Proc 38: 1320–1322, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Nijenhuis T, Hoenderop JG, Bindels RJ: Downregulation of Ca(2+) and Mg(2+) transport proteins in the kidney explains tacrolimus (FK506)-induced hypercalciuria and hypomagnesemia. J Am Soc Nephrol 15: 549–557, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Hopping BN, Erber E, Grandinetti A, Verheus M, Kolonel LN, Maskarinec G: Dietary fiber, magnesium, and glycemic load alter risk of type 2 diabetes in a multiethnic cohort in Hawaii. J Nutr 140: 68–74, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Ridaura R, Willett WC, Rimm EB, Liu S, Stampfer MJ, Manson JE, Hu FB: Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care 27: 134–140, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Shi ZM, Hu XS, Yuan BJ, Gibson R, Dai Y, Garg M: Association between magnesium : iron intake ratio and diabetes in Chinese adults in Jiangsu Province. Diabet Med 25: 1164–1170, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Song Y, Manson JE, Buring JE, Liu S: Dietary magnesium intake in relation to plasma insulin levels and risk of type 2 diabetes in women. Diabetes Care 27: 59–65, 2004 [DOI] [PubMed] [Google Scholar]
- 25.van Dam RM, Hu FB, Rosenberg L, Krishnan S, Palmer JR: Dietary calcium and magnesium, major food sources, and risk of type 2 diabetes in U.S. black women. Diabetes Care 29: 2238–2243, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Villegas R, Gao YT, Dai Q, Yang G, Cai H, Li H, Zheng W, Shu XO: Dietary calcium and magnesium intakes and the risk of type 2 diabetes: the Shanghai Women’s Health Study. Am J Clin Nutr 89: 1059–1067, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chambers EC, Heshka S, Gallagher D, Wang J, Pi-Sunyer FX, Pierson RN Jr: Serum magnesium and type-2 diabetes in African Americans and Hispanics: a New York cohort. J Am Coll Nutr 25: 509–513, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Corica F, Corsonello A, Ientile R, Cucinotta D, Di Benedetto A, Perticone F, Dominguez LJ, Barbagallo M: Serum ionized magnesium levels in relation to metabolic syndrome in type 2 diabetic patients. J Am Coll Nutr 25: 210–215, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Huerta MG, Roemmich JN, Kington ML, Bovbjerg VE, Weltman AL, Holmes VF, Patrie JT, Rogol AD, Nadler JL: Magnesium deficiency is associated with insulin resistance in obese children. Diabetes Care 28: 1175–1181, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Lima ML, Cruz T, Rodrigues LE, Bomfim O, Melo J, Correia R, Porto M, Cedro A, Vicente E: Serum and intracellular magnesium deficiency in patients with metabolic syndrome--evidences for its relation to insulin resistance. Diabetes Res Clin Pract 83: 257–262, 2009 [DOI] [PubMed] [Google Scholar]
- 31.de Lordes Lima M, Cruz T, Pousada JC, Rodrigues LE, Barbosa K, Canguçu V: The effect of magnesium supplementation in increasing doses on the control of type 2 diabetes. Diabetes Care 21: 682–686, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez-Morán M, Guerrero-Romero F: Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects: a randomized double-blind controlled trial. Diabetes Care 26: 1147–1152, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Augusto J-F, Subra J-F, Duveau A, Rakotonjanahary J, Dussaussoy C, Picquet J, Croue A, Villemain F, Onno C, Sayegh J: Relation between pretransplant magnesemia and the risk of new onset diabetes after transplantation within the first year of kidney transplantation. Transplantation, 97: 1155–1160,2014 [DOI] [PubMed] [Google Scholar]
- 34.Osorio JM, Bravo J, Pérez A, Ferreyra C, Osuna A: Magnesemia in renal transplant recipients: relation with immunosuppression and posttransplant diabetes. Transplant Proc 42: 2910–2913, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Van Laecke S, Desideri F, Geerts A, Van Vlierberghe H, Berrevoet F, Rogiers X, Troisi R, de Hemptinne B, Vanholder R, Colle I: Hypomagnesemia and the risk of new-onset diabetes after liver transplantation. Liver Transpl 16: 1278–1287, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Van Laecke S, Van Biesen W, Verbeke F, De Bacquer D, Peeters P, Vanholder R: Posttransplantation hypomagnesemia and its relation with immunosuppression as predictors of new-onset diabetes after transplantation. Am J Transplant 9: 2140–2149, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Garg N, Weinberg J, Ghai S, Bradauskaite G, Nuhn M, Gautam A, Kumar N, Francis J, Chen JL: Lower magnesium level associated with new-onset diabetes and pre-diabetes after kidney transplantation. J Nephrol 27: 339–344, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Van Laecke S, Nagler EV, Taes Y, Van Biesen W, Peeters P, Vanholder R: The effect of magnesium supplements on early post-transplantation glucose metabolism: a randomized controlled trial. Transpl Int 27: 895–902, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Pham PC, Pham PM, Pham SV, Miller JM, Pham PT: Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol 2: 366–373, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Barbagallo M, Dominguez LJ: Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome and insulin resistance. Arch Biochem Biophys 458: 40–47, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D American Diabetes Association : Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 28: 956–962, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Lecube A, Baena-Fustegueras JA, Fort JM, Pelegrí D, Hernández C, Simó R: Diabetes is the main factor accounting for hypomagnesemia in obese subjects. PLoS One 7: e30599, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tong GM, Rude RK: Magnesium deficiency in critical illness. J Intensive Care Med 20: 3–17, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Famure O, Phan NA, Kim SJ: Health information management for research and quality assurance: the Comprehensive Renal Transplant Research Information System. Healthc Manageme Forum 27: 30–36, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Feldman HI, Joffe M, Robinson B, Knauss J, Cizman B, Guo W, Franklin-Becker E, Faich G: Administration of parenteral iron and mortality among hemodialysis patients. J Am Soc Nephrol 15: 1623–1632, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Royston P, Ambler G, Sauerbrei W: The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol 28: 964–974, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M STROBE Initiative : Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology 18: 805––835., 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.