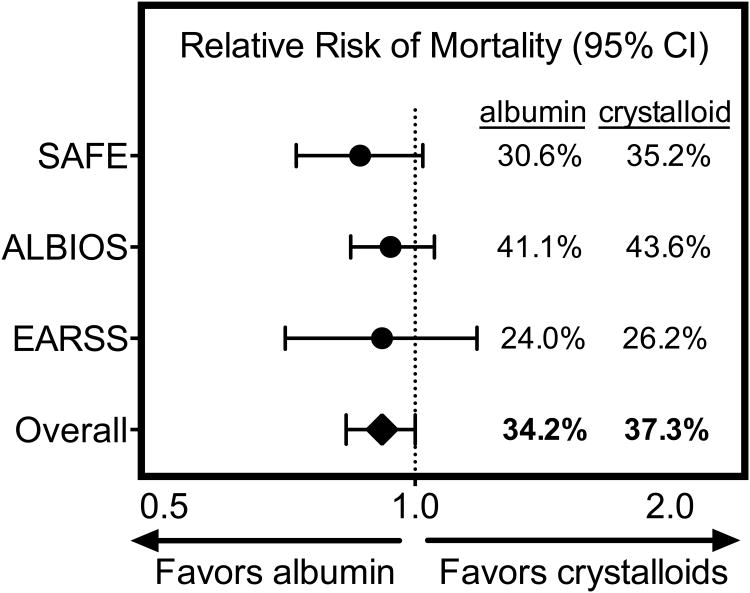
Figure 3. Mortality of sepsis patients in trials of albumin administration.
Relative risks of death by 28 days with albumin (n=603) versus saline (n=615) for patients with severe sepsis in the SAFE study36, death by 90 days with albumin (n=888) versus crystalloid (n=893) in the ALBIOS study37, and death by 28 days with albumin (n=399) versus saline (n=393) in the EARSS study38 are displayed with accompanying 95% confidence intervals.
Adapted from Wiedermann, C. J. & Joannidis, M. Albumin replacement in severe sepsis or septic shock. N. Engl. J. Med. 371, 83 (2014); with permission.