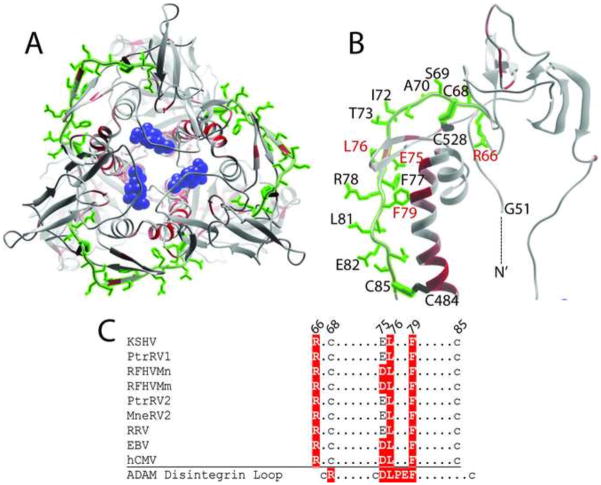
Figure 8.
Structural model and conservation of a disintegrin-like domain (DLD) in KSHV gB. A conserved DLD motif (aa66-85) identified previously in KSHV gB (Walker et al., 2014) was mapped onto the KSHV gB trimer (A) and monomer (B) model structures. The amino acid side chains in the motif were colored green and are shown in (B). The functional importance of each amino acid was determined by meta-functional signature (MFS) analysis and the scores were mapped onto the KSHV gB structure as a temperature gradient. Predicted disulfide linkages between C68/C528 and C85/C484 are shown. The N-terminal G51 of the ectodomain is indicated. C) Conservation of the DLD motif across the RV1 and RV2 rhadinovirus gB sequences compared to the homologous regions in EBV and hCMV gB. Residues conserved with the disintegrin loop of the ADAMS family, which bind to α9β1 integrin (Feire et al., 2004), are colored red (B) and highlighted in red (C). The NAG molecule bound to N628 is colored blue in B.