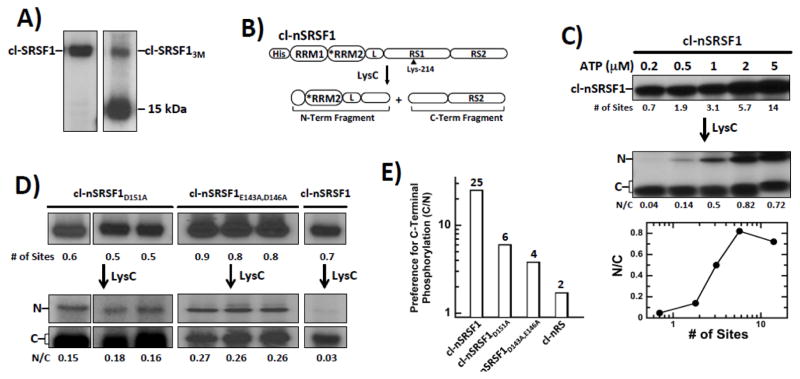
Figure 6. Residues in RRM2 Regulate Directional Phosphorylation of RS1 in SRSF1.
A) Phosphorylation of cl-SRSF1 & cl-SRSF13M monitored by autoradiography. A proteolytic fragment of cl-SRSF13M migrates at 15 kDa. B) N-terminal His-tagged cleavage substrate, cl-nSRSF1. LysC cleavage generates two fragments splitting RS1 in half. C) SRPK1 phosphorylates cl-nSRSF1 in a C-to-N manner. cl-nSRSF1 is phosphorylated by excess SRPK1 using varying 32P-ATP and then treated with LysC to generate the N- & C-terminal fragments. The ratios of the N- & C-terminal fragments (N/C) are plotted as a function of the total number of phosphorylation sites in cl-nSRSF1. D) N/Cs ratio of cl-SRSF1D151A and cl-SRSF1D143A,E146A phosphorylated with SRPK1 at low ATP (0.2 μM) and treated with LysC. E) Bar graph showing SRPK1 specificity (C/N ratio) for C-terminus of RS1 for cl-SRSF1, cl-SRSF1D151A, cl-SRSF1D143A,E146A & cl-nRS.