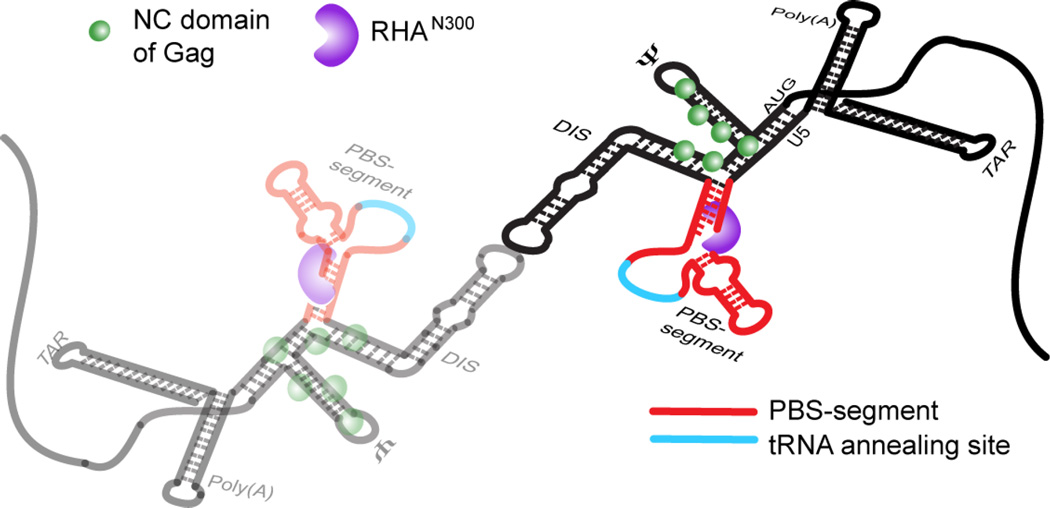
Fig. 6.
Model for the 1:1 stoichiometry of RHA bound to dimeric HIV-1 5′-UTR, which is spatially consistent with RHA’s known role in primer placement during initiation of reverse transcription. The RHA N-terminal double-stranded RNA-binding domains, shown in purple, are recruited to double-stranded residues in the PBS-segment, which is juxtaposition to CES-bound Gag p55 NC domain during assembly in virus producer cells. The PBS-segment is highlighted in red with the tRNALys3-annealing site highlighted in cyan; the rest of the 5′-UTR sequence is shown in black. The N-terminal domain of RHA binds the dimeric 5′-UTR independently of NC binding.