Abstract
Allergic asthma is characterized by Th2-driven eosinophilic airway inflammation and by a central feature called airway hyperreactivity (AHR), development of which requires the presence of classical type I invariant NK T (iNKT) cells. Allergen-induced AHR, however, develops in β2-microglobulin (β2m)−/− mice, which lack classical iNKT cells, suggesting that in some situations iNKT cells may be dispensable for the development of AHR. In contrast, our studies now suggest that a CD1d-restricted, NK1.1+ noninvariant TCR NKT cell population is present in β2m−/− mice and is responsible for the development of AHR but not for Th2 responses. Furthermore, treatment of β2m−/− mice with anti-CD1d mAb or anti-NK1.1 mAb unexpectedly abolished allergen-induced AHR. The CD1-restricted NKT cells in these mice, which failed to respond to α-galactosylceramide and which therefore were not classical type I iNKT cells, appear to represent an NKT cell subset restricted by a β2m-independent form of CD1d. These results indicate that, although classical type I iNKT cells are normally required for the development of AHR, under different circumstances other NKT cell subsets, including nonclassical NKT cells, may substitute for classical iNKT cells and induce AHR.
Asthma is an immunological disease that includes multiple inflammatory and clinical phenotypes characterized by symptoms of recurrent wheezing, coughing, and shortness of breath. A central and cardinal feature common to all forms of asthma is airway hyperreactivity (AHR),4 the heightened responsiveness of the airways to nonspecific stimuli (1). In allergic asthma, the most common form of asthma, the airways contain large numbers of eosinophils, allergen-specific CD4+ Th2 cells, as well as mast cells and basophils, but the development of allergen-induced AHR has been shown in a number of circumstances to require the presence of CD1d-restricted, classical, or type I NKT cells (2, 3). Classical NKT cells express a conserved or invariant TCR (iNKT cell) called Vα14Jα18 in mice or Vα24 in humans, recognize lipid Ags such as α-galactosylceramide presented by CD1d, and rapidly produce large quantities of IL-4 and IL-13 (4, 5). Allergen-induced AHR fails to develop in CD1d−/− mice, which lack the restriction element of NKT cells and therefore lack NKT cells, and fails to develop in Jα18−/− mice, which lack the conserved α-chain of the invariant TCR and therefore lack type I iNKT cells (2, 3, 6). Moreover, recent studies have shown that other forms of AHR induced by exposure to ozone or with pulmonary virus infection also require the presence of iNKT cells (7, 8). Since type I iNKT cells are increased in lungs of patients with severe asthma compared with that in normal individuals (8–10), we have hypothesized that type I iNKT cells play a critical role in regulating the development of at least several forms of asthma.
Classical type I iNKT cells represent only one of several subsets of NKT cells. For example, nonclassical type II NKT cells express a broad repertoire of (noninvariant) α and β TCR chains and although restricted by CD1d, type II NKT cells are not reactive with CD1d α-GalCer complexes. Nonclassical type II NKT cells are present in Jα18−/− mice, which fail to develop AHR (2, 3), indicating that under normal circumstances nonclassical type II NKT cells are not capable of inducing or are not involved in the development of AHR. Furthermore, because type II NKT cells are difficult to isolate, study, and specifically activate until now, the role of type II NKT cells in the development of AHR has not been specifically examined. However, type II NKT cells are involved in regulating the development of other inflammatory diseases, including hepatitis (11), some forms of cancer (12), and human inflammatory bowel disease (ulcerative colitis) (13).
To more clearly understand the role of NKT cells and specifically the role of type II NKT cells in the development of asthma, we examined β2-microglobulin-deficient (β2m−/−) mice in a mouse model of asthma. β2m−/− mice do not express class I MHC molecules or CD1d and are thought to lack both type I and type II NKT cells (as well as CD8+ T cells). However, β2m−/− mice develop robust AHR responses when sensitized and challenged with allergen (14, 15), suggesting that AHR, at least in some circumstances, may develop in the absence of NKT cells. In our current studies, however, we showed that although allergen-induced AHR responses did indeed occur in β2m−/− mice, surprisingly the AHR response was blocked by both an anti-CD1d mAb and by an anti-NK1.1 mAb, but not by anti-asialo GM1 Ab, which eliminates NK cells. The β2m−/− mice failed to develop AHR when challenged with α-GalCer, demonstrating the absence of type I iNKT cells. Rather, these mice appeared to contain type II CD1d-restricted, NK1.1+ NKT cells, restricted by a β2m-independent form of CD1d and capable of inducing AHR in the β2m−/− mice. These results together suggest that the development of AHR under different circumstances may require the presence of different forms or subpopulations of NKT cells, including type I, classical iNKT cells, as well as a subset of type II NKT cells.
Materials and Methods
Mice
Wild-type (WT) C57BL/6 mice and B6.129P2-B2mtm1Unc (β2m−/− ) mice on the C57BL/6 background were purchased from The Jackson Laboratory. Jα18−/− mice on the C57BL/6 background were a gift from M. Taniguchi (RIKEN, Yokohama, Japan) and T. Nakayama (Chiba University, Chiba, Japan). CD1d−/− mice on the C57BL/6 background were obtained from M. Exley (Beth Israel Deaconess Hospital, Harvard Medical School, Boston, MA). Animals were used between 6 and 11 wk of age and were age and sex matched. All animal protocols were approved by the Children’s Hospital Committee on animal welfare.
Induction of AHR and measurement of airway responsiveness
Mice were sensitized with 100 μg of OVA (Valeant Pharmaceuticals) adsorbed to 2 mg of an aqueous solution of alum i.p. on days 0 and 14, followed by 50 μg of OVA in 50 μl of PBS administered intranasally on days 14 and 25–27. Control mice received i.p. injections of PBS and intranasal administrations of normal saline. AHR was measured on day 28. AHR was assessed by invasive measurement of airway resistance, in which anesthetized and tracheostomized mice were mechanically ventilated using a described method (2). Aerosolized methacholine was administered for 20 breaths in increasing concentrations (saline, 1.25, 2.5, 5, and 10 mg/ml). We continuously computed lung resistance (RL) by fitting flow, volume, and pressure to an equation of motion. In some experiments, AHR was also assessed 24 h after glycolipid Ags were administered intranasally (2 μg of α-GalCer or 20 μg of Sphingomonas glycolipid Ag (PS-30), both in 50 μl) to mice anesthetized with isoflurane as described previously (16). α-GalCer was synthesized by P. B. Savage (Brigham Young University, Provo, UT). PS-30 (also known as PBS-30) was synthesized by P. B. Savage as previously described (17). We initially brought up the PS-30 Ag in 10% DMSO and PBS (4 μg/μl) and diluted to 1% DMSO before administration. Saline and 1% DMSO were used as controls for the α-GalCer and PS-30 challenges, respectively. In other experiments, CD1d−/− mice were reconstituted with NKT cell-enriched populations. These were prepared from the spleens of β2m−/− or WT mice depleted of B cells with anti-CD19 mAb by magnetic sorting. In brief, 2 × 107 purified splenic cells (~1.2 × 105 β2m−/− NKT cells or 3.2 × 105 WT NKT cells) with 106 bone marrow-derived dendritic cells were transferred i.v. into CD1d−/− mice on day 20, after sensitization on days 0 and 14 by immunization with OVA in alum. The mice were then challenged with OVA intranasally on days 21, 22, and 23 and analyzed for AHR on day 24.
Collection and analysis of bronchoalveolar lavage (BAL) fluid
Immediately after the AHR measurement, the trachea was cannulated, the lungs were lavaged two times with 0.5 ml of PBS, and the fluid was pooled. We counted and analyzed cells in BAL fluid as previously described (18). The relative number of different types of leukocytes was determined from slide preparations of BAL fluid stained with Diff-Quik solution (Dade Behring).
Restimulation of bronchial lymph node cells in vitro
Bronchial lymph node cells were obtained by mechanical disruption and were restimulated in vitro (5.0 × 105 cells/well in a round 96-well plate) with OVA. Supernatants were collected after 96 h and kept at −20°C until assayed to determine the secretion of cytokines in the cultures.
Lung and spleen cells isolation
Whole lungs were flushed with PBS injected into the right ventricle, removed, and rinsed in PBS. The lungs were then diced on a wax board before incubating in 9.6 ml of RPMI 1640 medium with 0.1% DNase I (fraction IX; Sigma-Aldrich) and 1.6 mg/ml collagenase (CSL4; Worthington Biochemicals) at 37°C on an orbital shaker for 30 min. The digest was passed multiple times through an 18-gauge needle and allowed to incubate for another 30 min before being filtered. RBC were removed by 4-min incubation in lysis buffer (Sigma-Aldrich) at room temperature. Single-cell suspensions of spleen lymphocytes were obtained by mechanical disruption and RBC lysis as above.
Flow cytometry for cell surface markers
We collected cells from BAL fluid, spleen, or lungs and stained with anti-CD45-PE-Cy5.5 (eBioscience), anti-CD45-PE-Texas Red (Caltag Laboratories), anti-TCRβ-allophycocyanin (eBioscience), anti-NK1.1-FITC (eBioscience), α-GalCer-loaded CD1d tetramer-PE (National Institute of Allergy and Infectious Disease Tetramer Facility), CD8α-Alexa Fluor 700 (Caltag Laboratories), anti-CD49b-FITC (DX5; BD Pharmingen), anti-TCRγδ-PE-Cy5.5 (eBioscience), and CD4-Alexa Fluor 750 (eBioscience). Control samples were labeled with isotype-matched control Abs (mouse IgG2b-FITC (BD Pharmingen) for anti-NK1.1-FITC) and unloaded tetramer-PE (National Institute of Allergy and Infectious Disease Tetramer Facility)). Analytical flow cytometry was done on a FACSCanto instrument (BD Biosciences). Analysis was performed with FlowJo software (Tree Star).
Detection of intracellular cytokines
Flow cytometric measurements of cytokine production in NKT cells were done as described previously (2). In brief, we stimulated NKT cells in some experiments with PMA (20 ng/ml) and ionomycin (500 ng/ml) for 3 h. Fixation and permeabilization were done on collected cells using Cytofix/Cytoperm and Perm/Wash (BD Pharmingen) according to the manufacturer’s instructions. Cytoplasmic IL-4, IL-13, and IFN-γ were stained with anti-IL-4-PE (Caltag Laboratories), anti-IL-13-PE (eBioscience), and anti-IFN-γ-PE (eBioscience). Control samples were labeled with isotype-matched control Ab (rat IgG1-PE; eBioscience).
Measurements of cytokines and OVA-specific serum IgE
ELISA for IL-4 assays were performed as described previously (18). The Ab pairs used were as follows, listed by capture/biotinylated detection: BVD4-1D11/BVD6-24G2. The standards were recombinant cytokine curves generated in 1/2 dilutions from 500 to 7.5 pg/ml. For the OVA-specific IgE assay, mice were bled and the OVA-specific IgE Abs were measured in serum using a modified OVA-specific ELISA as described previously (19).
NK1.1+ cell and NK cell depletion and CD1d blockade
NK1.1+ cells were depleted via a single i.p. injection of 500 μg of anti-NK1.1 mAb (PK136; mouse IgG2a) 4 days before the first intranasal OVA challenge. CD1d was blocked by two i.p. injections per week of 500 μg of β2m-independent anti-CD1d mAb (3C11; rat IgM, a gift from M. Exley, Beth Israel Deaconess Medical Center, Harvard Medical School) or another anti-CD1d mAb (HB323) from 1 day before the start of sensitization through 1 day before the first intranasal OVA challenge. NK cells were depleted by treatment with anti-asialo GM1 Ab (Wako Chemicals) administered (0.025 ml i.v.) to β2m−/− mice on days −1, 4, 9, 13, 18, and 22. These mice were sensitized with OVA on days 7 and 14 and challenged with OVA on days 21, 22, and 23. PK136, 3C11, HB323, and isotype mouse IgG2a mAbs were purified by ammonium sulfate precipitation and dialysis from ascites produced by hybridoma cells, which had been sub-cloned by limiting dilution. Isotype control rat IgM mAb was obtained from Millipore and dialyzed against PBS before use.
CFSE proliferation of NKT cells induced by B cells
NK1.1+ cells were isolated from spleens of WT and β2m−/− mice by incubation with anti-NK1.1 mAb and anti-biotin MACS beads (Miltenyi Biotec) using an autoMACS according to the manufacturer’s instructions. Purified NK1.1+ cells were labeled with CFSE (4 μM/ml). B cells from WT and β2m−/− mice were purified using anti-IgG plus IgM (H + L; Jaxson Research)-coated plates and stimulated with LPS (10 μg/ml) for 24 h. For proliferation, NK1.1+ cells (105 cells/well) were cultured either with 3C11 anti-CD1d mAb (10 μg/ml) or isotype control mAb (10 μg/ml) in the presence of LPS-stimulated B cells (104 cells/well). To measure proliferation, CFSE dilution was determined by FACS analysis after 72 h.
Statistical analysis
Nonlinear regression analyses were used to compare airway resistances between groups (Prism 4; GraphPad software). Otherwise, unpaired Student’s t tests were used for comparisons between groups (SPSS 14). A value of p < 0.05 was considered significant.
Results
β2m−/− mice develop allergen-induced AHR, airway inflammation and Th2 responses
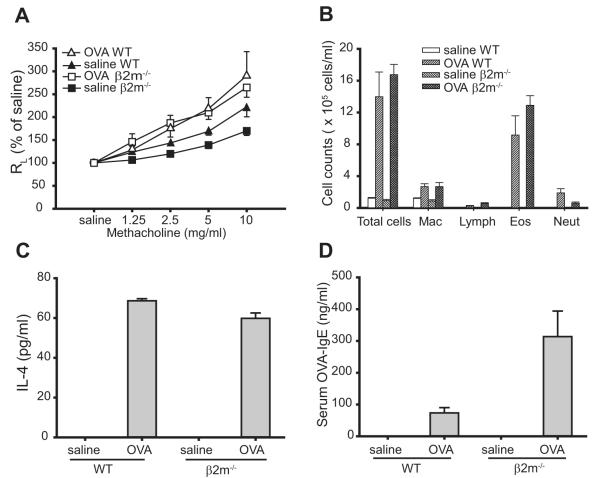
β2m−/− mice sensitized and challenged with OVA developed significant AHR (p < 0.0001, compared with saline-treated controls) comparable to AHR responses induced in WT C57BL/6 mice (Fig. 1A). In both β2m−/− and WT mice, OVA sensitization and challenge significantly increased the number of cells in the BAL fluid compared with saline control mice, with eosinophils comprising the majority of cells (Fig. 1B). OVA-specific CD4+ Th2 responses developed normally in β2m−/− and in WT mice, as OVA stimulation of bronchial lymph node cells from OVA-immunized β2m−/− and WT mice induced IL-4 production (Fig. 1C). In addition, OVA-specific IgE levels in serum were increased in OVA-immunized β2m−/− and WT mice, but not in control saline-treated mice (Fig. 1D). These data indicate that β2m−/− mice develop allergen-induced AHR with eosinophilic airway inflammation and OVA-specific Th2 and IgE responses that were equivalent or more severe than those observed in WT C57BL/6 mice.
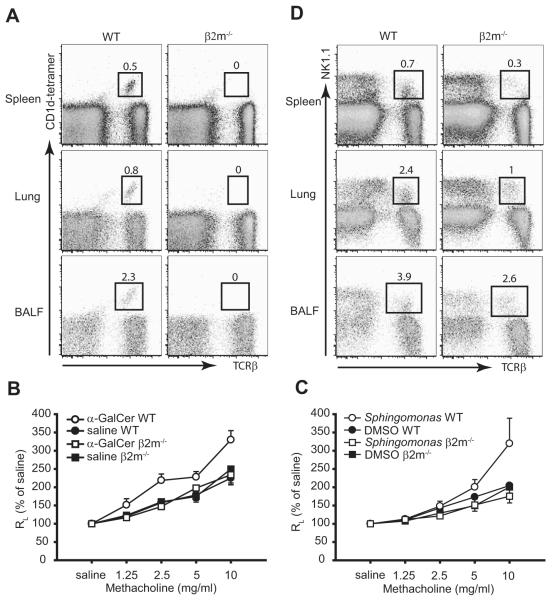
FIGURE 1.
Development of allergen-induced AHR, airway inflammation, and Th2 responses in β2m−/− mice. A, β2m−/− mice develop allergen-induced AHR. AHR was determined by invasive measurement of airway resistance (RL) in response to increasing doses of methacholine in sensitized mice 24 h after the last OVA challenge. Data represent the mean ± SEM percentage of saline value from three experiments (n = 9–16). Values of p are 0.019 for OVA WT vs saline WT and <0.0001 for OVA β2m−/− vs saline β2m−/−. B, β2M−/− mice induce airway eosinophilia. BAL fluid from mice in A was analyzed immediately after AHR measurement. Data represent the number of cells per milliliter in BAL fluid and are the mean ± SEM, representative of three experiments. Mac, Macrophage; Lymph, lymphocytes; Eos, eosinophils; Neut, neutrophils. C, β2m−/− mice induce OVA-induced IL-4 production from bronchial lymph node T cells. Bronchial lymph nodes from mice in A were removed and analyzed immediately after AHR measurement. IL-4 was measured by ELISA in supernatants from the lymph node cell cultures stimulated with 25 μg/ml OVA. Data are the mean ± SEM, representative of three experiments. D, β2m−/− mice produce OVA-specific serum IgE. OVA-specific IgE was measured by ELISA in sera taken from mice in A, immediately after AHR measurement. Data are the mean ± SEM, representative of three experiments.
Blockade of CD1d in β2m−/− mice aborts allergen-induced AHR
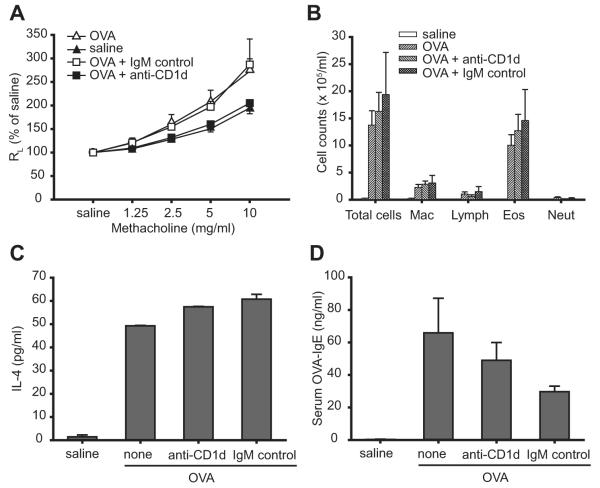
To determine the role of CD1d in the development of allergen-induced AHR in β2m−/− mice, β2m−/− mice were treated with anti-CD1d-blocking mAb before sensitization and challenge with OVA. The anti-CD1d mAb used, 3C11, has been shown to recognize CD1d molecules that may form in the absence of β2m (20). Treatment with anti-CD1d mAb significantly blocked the development of OVA-induced AHR, compared with treatment with no Ab (p = 0.001) or with an isotype control mAb (p < 0.0001; Fig. 2A). However, treatment with anti-CD1d mAb did not decrease the number of total cells and eosinophils in BAL fluids (Fig. 2B), OVA-induced IL-4 concentrations by bronchial lymph node cells (Fig. 2C), and serum OVA-specific IgE levels (Fig. 2D). Allergen-induced AHR in β2m−/− mice was also blocked by another anti-CD1d mAb, HB323 (data not shown). These data strongly suggest that β2m-independent CD1d-restricted NKT cells are required for the development of allergen-induced AHR in β2m−/− mice, but that eosinophilic airway inflammation and OVA-specific Th2 responses occurred independently of NKT cells, possibly as a result of OVA-specific Th2 cell development.
FIGURE 2.
Blockade of CD1d in β2m−/− mice inhibits the development of allergen-induced AHR. A, Treatment with anti-CD1d mAb, but not isotype control mAb, blocks the development of AHR in β2m−/− mice. β2m−/− mice were treated with the 3C11 anti-CD1d or isotype control mAb as described in Materials and Methods and AHR was measured as in Fig. 1A. Data represent the mean ± SEM percentage of saline value from four experiments (n = 4–18). Values of p are 0.001 for OVA + anti-CD1d vs OVA and <0.0001 for OVA + anti-CD1d vs OVA + IgM control. B, Treatment with anti-CD1d mAb does not inhibit the development of eosinophilic airway inflammation in β2m−/− mice. BAL fluid from mice in A, was analyzed as in Fig. 1B. C, Treatment with anti-CD1d mAb does not inhibit OVA-induced IL-4 production from bronchial lymph node T cells in β2m−/− mice. Bronchial lymph nodes from mice in A were removed and analyzed as in Fig. 1C. Lymph node cell cultures were stimulated with 100 μg/ml OVA. D, Treatment with anti-CD1d mAb does not inhibit OVA-specific serum IgE production in β2m−/− mice. OVA-specific IgE from mice in A was measured as in Fig. 1D. See Fig. 1 legend for definitions of abbreviations.
Depletion of NKT cells in β2m−/− mice eliminates OVA-induced AHR
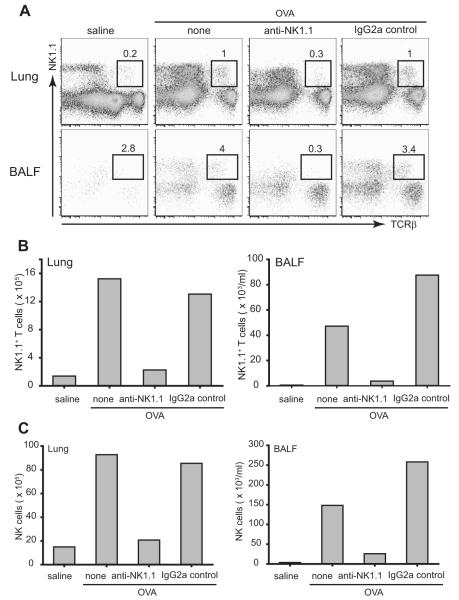
To confirm the requirement of NKT cells for the development of allergen-induced AHR, we depleted NKT cells from lung and airways of β2m−/− mice using anti-NK1.1 mAb before assessment of AHR. In vivo treatment with anti-NK1.1 mAb but not isotype control mAb, 4 days before the first OVA challenge, completely depleted NK1.1+ T cells from OVA-immunized lung and BAL fluids in β2m−/− mice (Fig. 3, A and B). Splenic NKT cells were also depleted after treatment with anti-NK1.1 mAb in β2m−/− mice (data not shown). In addition, NK cells (NK1.1+ TCRβ− cells) were substantially reduced in lung (Fig. 3, A and C), BAL fluids (Fig. 3, A and C), and spleen (data not shown) after treatment with anti-NK1.1 mAb. The depletion of the NKT cells by anti-NK1.1 mAb was also shown by staining of NKT cells with DX5 mAb rather than anti-NK1.1 mAb. Thus, DX5+TCRβ+ NKT cells and DX5+ NK cells were substantially reduced in lung and BAL fluids after treatment with anti-NK1.1 mAb in β2m−/− mice (data not shown).
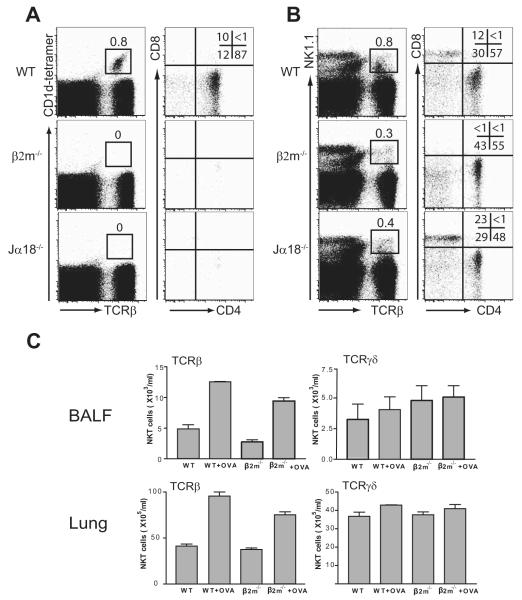
FIGURE 3.
Depletion of NKT cells from lung and airways of β2m−/− mice after treatment with anti-NK1.1 mAb. β2m−/− mice were treated with anti-NK1.1 or isotype control mAb as described in Materials and Methods. A, Lung and BAL fluid (BALF) cells were obtained and pooled 24 h after the last OVA challenge (n = 3) and stained with anti-NK1.1 mAb and anti-TCRβ mAb for evaluation by flow cytometry. Treatment with anti-NK1.1 mAb, but not isotype control mAb, in β2m−/− mice substantially reduced the number of NK1.1+TCRβ+ NKT cells in the lungs and BAL fluid. The number of NKT cells are shown as a percentage of total lymphocytes. B, The absolute number of NKT cells in the lungs and BAL fluid was calculated, and shows that treatment with anti-NK1.1 mAb greatly reduced the number of NKT cells in these compartments. Data are representative of three experiments. C, Depletion of NK cells from lung and airways of β2m−/− mice after treatment with anti-NK1.1 mAb. β2m−/− mice were treated with anti-NK1.1 or isotype control mAb as described in Materials and Methods. Lung and BAL fluid cells were obtained and pooled 24 h after the last OVA challenge (n = 3) and stained with anti-NK1.1 mAb and anti-TCRβ mAb for evaluation by flow cytometry. The proportion of NK1.1+TCRβ− NK cells was obtained and the total cell counts were calculated. Treatment with anti-NK1.1 mAb, but not isotype control mAb, in β2m−/− mice substantially reduced the total counts of NK cells from lung (left) and airways (right), which is representative of three experiments.
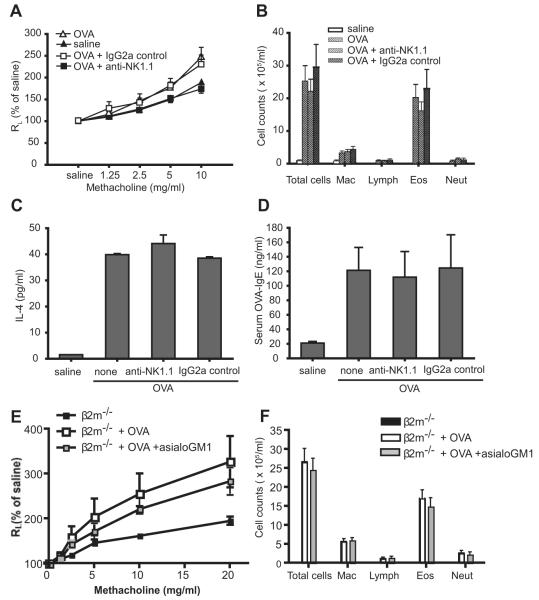
Importantly, treatment of the β2m−/− mice with anti-NK1.1 mAb, which removes NKT cells and NK cells, abolished the development of allergen-induced AHR in β2m−/− mice, compared with treatment with no (p < 0.0001) or isotype control mAb (p < 0.0001; Fig. 4A). However, depletion of the NK1.1+ cells did not decrease the total number of cells and eosinophils in BAL fluid (Fig. 4B), OVA-induced IL-4 productions by bronchial lymph node cells (Fig. 4C), and serum OVA-specific IgE levels (Fig. 4D). To demonstrate that the depletion of NK cells by anti-NK1.1 mAb did not affect the AHR response in these mice, we showed that treatment of the β2m−/− mice with anti-asialo GM1 Ab, which depletes only NK cells, did not reduce AHR (Fig. 4E) nor airway eosinophilia (Fig. 4F). These results confirmed that CD1d-restricted NKT cells, but not NK cells, were essential for the development of allergen-induced AHR in β2m−/− mice, but not for the eosinophilic airway inflammation and CD4+ Th2 responses.
FIGURE 4.
Depletion of NKT cells in β2m−/− mice aborts the development of allergen-induced AHR. A, Treatment with anti-NK1.1 mAb, but not isotype control mAb, inhibits the development of AHR in β2m−/− mice. β2m−/− mice were treated with anti-NK1.1 mAb as described in Materials and Methods and AHR was measured as in Fig. 1A. Data represent the mean ± SEM percentage of saline values from four experiments (n = 10–14). The value of p is <0.0001 for OVA + anti-NK1.1 vs OVA or OVA + IgG2a control. B, Treatment with anti-NK1.1 mAb does not inhibit the development of eosinophilic airway inflammation in β2m−/− mice. BAL fluid from mice in A, was analyzed as in Fig. 1B. C, Treatment with anti-NK1.1 mAb does not inhibit OVA-induced IL-4 production from bronchial lymph node T cells in β2m−/− mice. Bronchial lymph nodes from mice in A, were removed and analyzed as in Fig. 1C. Lymph node cell cultures were stimulated with 50 μg/ml OVA. D, Treatment with anti-NK1.1 mAb does not inhibit OVA-specific serum IgE production in β2m−/− mice. OVA-specific IgE from mice in A, was measured as in Fig. 1D. E, Treatment with anti-asialo GM1 Ab to deplete NK cells does not block the development of OVA-induced AHR in β2m−/− mice. β2m−/− mice were treated with anti-asialo GM1 Ab during the sensitization and challenge phases. AHR was assessed in intubated and mechanically ventilated mice. F, Treatment with anti-asialo GM1 Ab does not block the development of OVA-induced airway inflammation in β2m−/− mice. BAL fluid was taken from mice treated as described in E. See Fig. 1 legend for definitions of abbreviations.
β2m−/− mice have type II, but not type I iNKT cells
Since the development of allergen-induced AHR has been shown to require the presence of type I iNKT cells (2, 3), we examined the β2m−/− mice for the presence of type I iNKT cells. Although CD1d-tetramer-binding type I iNKT cells were clearly observed in spleen, lung, and BAL fluid of OVA-immunized WT C57BL/6 mice, no type I NKT cells were detected in OVA-immunized β2m−/− mice (Fig. 5A). Furthermore, although intranasal administration α-GalCer, which specifically activates type I iNKT cells, induced AHR in WT C57BL/6 mice, α-GalCer failed to induce AHR in β2m−/− mice (Fig. 5B). In addition, another glycolipid, a constituent of Sphingomonas cell membranes synthesized as PS-30, activates type I iNKT cells to induce AHR after intranasal administration (16). This glycolipid PS-30 failed to induce AHR in β2m−/− mice, but induced significant AHR in WT C57BL/6 mice (Fig. 5C). These data indicate that β2m−/− mice do not have type I iNKT cells in lung and airways. However, OVA-immunized β2m−/− mice contained a population of type II NKT cells in spleen, lung, and BAL fluids, as defined by expression of NK1.1 and TCRβ. The number of NK1.1+, TCR+, NKT cells, however, was approximately one-half of the number present in WT C57BL/6 mice (Fig. 5D), but included 2.6% of the lymphocytes in the BAL fluid, following sensitization and challenge with OVA. These data indicate that β2m−/− mice clearly have type II NKT cells but no type I iNKT cells and suggest that these type II NKT cells are capable of inducing AHR in these mice.
FIGURE 5.
β2m−/− mice have type II NKT cells but not type I iNKT cells. A, β2m−/− mice have no CD1d-tetramer+ type I iNKT cells. β2m−/− mice were sensitized and challenged with OVA. Spleen, lung, and BAL fluid (BALF) cells were obtained 24 h after the last OVA challenge and stained with α-GalCer-loaded CD1d tetramers and anti-TCRβ mAb, which are representative of at least three experiments. The number of CD1d-tetramer+ type I iNKT cells are shown as a percentage of total lymphocytes. B, β2m−/− mice do not respond to intranasal challenge of α-GalCer. Invasive measurement of airway resistance (RL) was performed 24 h after 2 μg of intranasal challenge of α-GalCer. Data are the mean ± SEM percentage of saline values from two experiments (n = 10–11). C, β2m−/− mice do not respond to intranasal challenge of Sphingomonas glycolipid Ag. AHR was measured as in B 24 h after 20 μg of intranasal challenge of Sphingomonas glycolipid Ag. Data are the mean ± SEM percentage of saline values (n = 4–5). D, β2m−/− mice have NK1.1+ T cells. Spleen, lung, and BAL fluid cells were obtained as in A and stained with anti-NK1.1 mAb and anti-TCRβ mAb. The number of NK1.1+ T cells is shown as a percentage of total lymphocytes.
Phenotypic and functional characteristics of NKT cells in β2m−/− mice
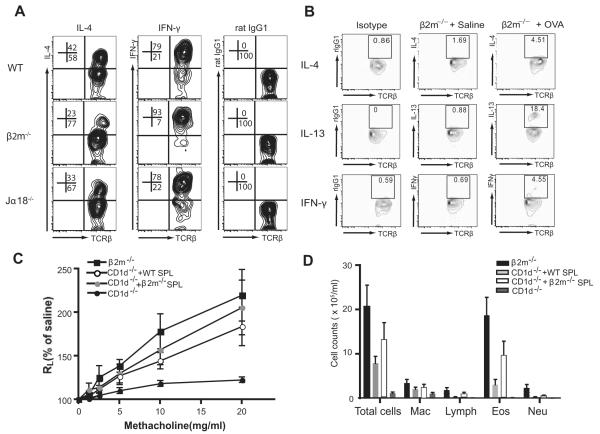
We compared the phenotype of NKT cells in β2m−/− mice with that in WT C57BL/6 and Jα18−/− mice. As discussed earlier, CD1d tetramer-staining type I NKT cells were present in WT but not in β2m−/− or Jα18−/− mice (Fig. 6A), but all three strains contained NK1.1+TCR+ NKT cells (Fig. 6B). In β2m−/− mice, NKT cells were CD4+ or CD4−CD8− double negative (DN) (no CD8+ cells present), while in WT and in Jα18−/− mice the NKT cells were CD4+, CD8+, and DN (Fig. 6B). The NK1.1+ cells in the lungs and BAL fluid of the β2m−/− mice sensitized and challenged with OVA expressed αβ and not γδ TCRs (Fig. 6C). Furthermore, the splenic NKT cells from WT, β2m−/−, and Jα18−/− mice produced IL-4 and IFN-γ by intracellular cytokine staining after activation with PMA and ionomycin (Fig. 7A), indicating that at least some of the type II NKT cells present in β2m−/− mice had an unrestricted cytokine profile. Moreover, the pulmonary NKT cells from β2m−/− mice sensitized and challenged with OVA spontaneously produced (without further activation) IL-13, but not much IL-4 and IFN-γ (Fig. 7B). These results indicate that IL-13 production from pulmonary type II NKT cells may contribute to the development of allergen-induced AHR in the β2m−/− mice. Finally, adoptive transfer of an NKT cell-enriched population purified from β2m−/− mice (or from WT mice) into NKT cell-deficient CD1d−/− mice rescued AHR (Fig. 7C) and airway inflammation (Fig. 7D). In these experiments, bone marrow-derived dendritic cells from WT mice were also transferred with the NKT cells to provide APC function. These studies indicate that the type II NKT cells in β2m−/− mice indeed function to induce AHR and airway inflammation.
FIGURE 6.
Characteristics of NKT cells in β2m−/− mice. A, Type I iNKT cells present in WT C57BL/6 mice are CD4+ and CD4−CD8− DN. Spleen cells were isolated from naive mice and stained with α-GalCer-loaded CD1d tetramers, anti-TCRβ mAb, anti-CD4 mAb, and anti-CD8α mAb. CD1d-tetramer+ type I iNKT cells were gated (left column; the number is shown as a percentage of total lymphocytes) and further analyzed for CD4 and CD8 expression (right column), representative of three experiments. B, NKT cells in β2m−/− mice are CD4+ and DN. Spleen cells were isolated as in A and stained with anti-NK1.1mAb,anti-TCRβmAb,anti-CD4 mAb, and anti-CD8α mAb. NK1.1+ T cells were gated (left column; the number is shown as a percentage of total lymphocytes) and further analyzed for CD4 and CD8 expression (right column), representative of three experiments. C, NKT cells in β2m−/− mice express primarily αβ and not γδ TCRs. Lung and BAL fluid (BALF) cells were obtained 24 h after the last OVA or saline challenge in β2m−/− mice and stained with anti-NK1.1 mAb and anti-TCRγδ mAb. The number of NK1.1+TCRγδ+ NKT cells is shown as the absolute number in BAL fluid and lung. Lung and BALF NK1.1+TCRγδ+ NKT cells were not increased in OVA-immunized β2m−/− mice compared with that in saline-treated control mice.
FIGURE 7.
Cytokine production in NKT cells from β2m−/− mice. A, NKT cells in β2m−/− mice produce IL-4 cytokine. Spleen cells were isolated as in Fig. 6A, stimulated with PMA and ionomycin, and stained with anti-NK1.1 mAb, anti-TCRβ mAb, and mAbs against IL-4 or IFN-γ, which are representative of three experiments. The numbers are shown as a percentage of NK1.1+ T cells. B, Spontaneous cytokines production from lung NKT cells of OVA-immunized β2m−/− mice. Lung cells were obtained 24 h after the last OVA or saline challenge in β2m−/− mice. Lymphocytes were enriched, cultured for 3 h without further stimulation, and stained with anti-NK1.1 mAb, anti-TCRβ mAb, and mAbs against IL-4, IL-13, or IFN-γ. The numbers shown represent the percentage of NK1.1+ T cells after gating on NK1.1+ cells. Lung NKT cells from OVA-immunized β2m−/− mice produced more IL-13, but not much IL-4 and IFN-γ, compared with saline-treated β2m−/− mice. C, Adoptive transfer of a NKT cell-enriched population from β2m−/− mice rescues AHR in NKT cell-deficient CD1d−/− mice. In brief, 2 × 107 spleen cells enriched for NKT cells from WT or β2m−/− mice were adoptively transferred into OVA-sensitized CD1d−/− mice. The recipients were then challenged with OVA intranasally and analyzed for AHR. D, BAL fluid was taken from the mice described in C and evaluated for cell type. See Fig. 1 legend for definitions of abbreviations.
NKT cell proliferation in β2m−/− mice caused by β2m-independent CD1d
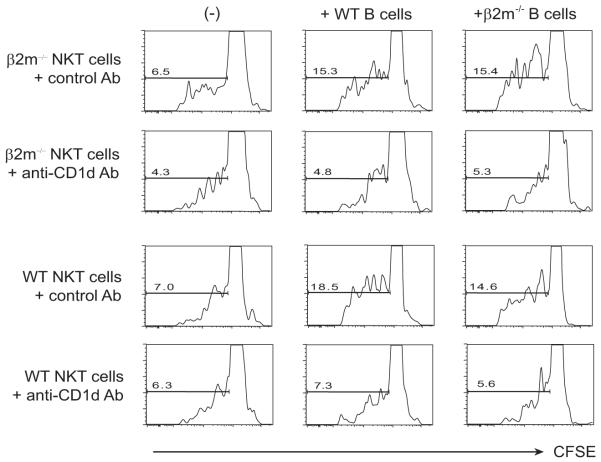
Based on our results, we hypothesized that AHR in the β2m−/− mice was caused by a NKT cell population that was restricted by a β2m-independent form of CD1d. This was confirmed by the fact that NKT cells purified from β2m−/− mice (or WT mice) proliferated when stimulated with B cells from β2m−/− mice (activated with LPS to induce expression of CD1d; Fig. 8, third column). This NKT cell proliferative response induced with B cells from β2m−/− mice was inhibited by anti-CD1d mAb (3C11) (Fig. 8, second row) but not isotype control mAb (Fig. 8, first row). These results provide very strong evidence for the existence of this NKT cells population (NK1.1+) in β2m−/− mice that is restricted by a β2M-independent form of CD1d.
FIGURE 8.
NKT cell proliferation in β2m−/− mice is caused by β2m-independent CD1d. Spleen NK1.1+ cells and LPS-stimulated spleen B cells were obtained from WT and β2m−/− mice as described in Materials and Methods and cultured in the presence of 3C11 anti-CD1d mAb or isotype control mAb. The NKT cells were labeled with CFSE, and B cell-induced proliferation was measured by staining and gating on TCRβ+ NKT cells and assessing CFSE expression. Treatment with anti-CD1d mAb, but not isotype control mAb, inhibited the proliferation of NKT cells induced by B cells from β2m−/− mice and WT mice.
Discussion
In these experiments, we demonstrated for the first time that a subset of type II NKT cells expressing noninvariant TCRs can potently induce AHR. Up to now, only type I iNKT cells and not type II NKT cells have been shown to be involved in the induction of AHR (2, 3). The type II NKT cells in β2m−/− mice, which developed severe AHR, expressed NK1.1 and TCRs (therefore by definition these are NKT cells), but do not respond to α-GalCer and therefore by definition are not type I iNKT cells. Furthermore, the development of AHR in β2m−/− mice was surprisingly blocked both by anti-CD1d mAb and by anti-NK1.1 mAb, but not by depletion of NK cells. Moreover, purified NKT cells from β2m−/− mice proliferated in vitro to activated B cells from β2m−/− mice in a CD1d-dependent fashion. Finally, adoptive transfer of a NKT cell-enriched population from β2m−/− mice into NKT cell-deficient CD1d−/− mice rescued AHR and airway inflammation. Together these results strongly suggest that AHR responses that develop in β2m−/− mice are caused by a unique subset of NKT cells that express noninvariant TCRs and are restricted by a β2M-independent form of CD1d.
The precise molecular structure of CD1d in β2m−/− mice is not entirely clear, since CD1d normally forms in association with β2m (21). However, several previous studies have demonstrated that cells transfected with CD1d express in the endoplasmic reticulum and on the cell surface a 45-kDa form of CD1d, independent of β2m (20, 22–24). Furthermore, human intestinal epithelial cells express CD1d in the absence of β2m, suggesting that β2m-independent CD1d-restricted type II NKT cells are normally present in humans (23). In our studies, the development of AHR was blocked by several anti-CD1d mAbs, including the 3C11 anti-CD1d mAb, which has been shown previously to block Ag presentation by β2m-independent CD1d (20) and the HB323 anti-CD1d mAb. Finally, the proliferation of purified NKT cells from β2m−/− mice to activated B cells from β2m−/− mice was blocked by the anti-CD1d mAb. Our studies therefore demonstrate for the first time that β2m-independent CD1d-restricted NKT cells can be pathogenic and responsible for the induction of AHR in the β2m−/− mice. What glycolipid Ags bind to and are presented by the β2m-independent form of CD1d to activate the type II NKT cells in β2m−/− mice are not yet clear, but these glycolipids are likely to be distinct from those that bind to the β2m-dependent form of CD1d.
Our current results demonstrating the role of CD1d- restricted type II NKT cells in causing AHR in β2m−/− mice explain the paradox of β2m−/− mice and AHR. Because β2m−/− mice were initially thought not to express CD1d and therefore not to contain any CD1d-restricted type I or type II NKT cells, the development of severe AHR responses in β2m−/− mice (14, 15) was perplexing, and suggested that NKT cells were not essential for the development of AHR. However, in this study, we demonstrated that β2m−/− mice do indeed contain a subset of NKT cells and provide strong evidence that (β2m independent)-CD1d restricted NKT cells are responsible for the allergen-induced AHR responses in these mice. Although recent studies have also demonstrated the presence of NKT cells in β2m−/− mice (25, 26), we now show that the NKT cells in β2m−/− mice are CD1d restricted and on adoptive transfer restore AHR responses in CD1d−/− but not Jα18−/− mice. These results suggest that the type II NKT cells in β2m−/− mice may develop unique functions distinct from those in Jα18−/− mice, such as the ability to induce AHR, possibly due to the absence of CD8+ T cells in the β2m−/− mice.
Previously, CD1d-restricted type II NKT cells have been shown to play important pathogenic roles in several other disease states. For example, in ulcerative colitis in humans, type II NKT cells are present in the lamina propria of the colon, produce IL-13 and IL-5, and are cytotoxic against intestinal epithelial cells (13), suggesting that type II NKT cells play a major effector cell role in the pathogenesis of ulcerative colitis. In addition, in a transgenic mouse model of acute hepatitis B infection, type II NKT cells have been shown to cause liver cell injury (11). Furthermore, type II NKT cells regulate autoimmune diabetes in a TCR-transgenic mouse (27) and down-regulate tumor-immune surveillance in mouse models of cancer by enhancing TGF-β production (28, 29). These studies thus demonstrated a pathogenic role for type II NKT cells, which may be related to the type II NKT cells responsible for AHR.
In our studies, we noted a separation between AHR and airway inflammation in that blockade of NKT cell function in the β2m−/− mice prevented AHR but not eosinophilic airway inflammation. These results are consistent with previous observations indicating that the presence of airway inflammation is neither sufficient nor necessary for the development of AHR (30, 31). Importantly, AHR rather than airway inflammation is arguably the critical feature in asthma responsible for clinical symptoms observed in patients with asthma (32). In any case, our results indicate that the mechanisms responsible for AHR are distinct from those responsible for the development of airway inflammation. We believe that NKT cells (type I or type II in different situations) are uniquely involved in the induction of AHR, whereas CD4+ Th2 cells independently drive the eosinophilic inflammation in the airways and IgE production, although NKT cells may also induce some amount of airway inflammation independent of conventional CD4+ T cells (1, 16). Thus, treatment with anti-CD1d or anti-NK1.1 mAbs, while blocking NKT cell function, did not affect IL-4 production from OVA-specific Th2 cells in bronchial lymph node cells.
The specific mechanisms by which type I and type II NKT cells, but not Th2 cells induce AHR, are not yet clear. Type I and type II NKT cells as well as Th2 cells all produce Th2 cytokines, but Th2 cells in the absence of NKT cells cannot induce AHR (2, 3). Explanations for this include the possibility that NKT cells produce additional cytokines that may be responsible for AHR, that NKT cells produce larger quantities of IL-4/IL-13 than Th2 cells, or that Ag-specific Th2 cells may not be present in sufficient quantity to induce AHR in the absence of NKT cells. Nevertheless, it should be noted that another type I NKT cell subset producing IL-17 (33, 34) has been shown to be required for the development of AHR induced by exposure to ozone, a major component of air pollution (7). The ozone-induced form of AHR was associated with airway neutrophils rather than eosinophils and could occur in the complete absence of adaptive immunity, consistent with the idea that NKT cells rather than eosinophils or Th2 cells may be the common denominator required for the development of AHR. Clearly, much more must be learned about various subsets of NKT cells and Th cells to more completely understand how AHR is regulated.
Treatment of β2m−/− mice with anti-NK1.1 mAb depleted both NKT and NK cells and abolished AHR, suggesting that NK cells might contribute to the development of AHR. Previous studies have suggested that NK cells might be involved in the development of airway eosinophilia. Importantly, however, in those studies, AHR responses were not evaluated and therefore a role of NK cells in AHR could not be assessed (35, 36). In contrast, in our studies, we found that treatment with anti-CD1d mAb produced the same loss of AHR as did treatment with anti-NK1.1 mAb. Moreover, treatment with anti-asialo GM1 Ab to specifically remove NK cells had no effect on OVA-induced AHR in the β2m−/− mice. These results together suggest that NKT cells and not NK cells were responsible for the development of AHR in the β2m−/− mice.
In summary, β2m−/− mice developed allergen-induced AHR, which was abolished by treatment with anti-CD1d or anti-NK1.1 mAb, but not with anti-asialo GM1 Ab. We found that the β2m−/− mice contained a novel β2m-independent CD1d-restricted type II NKT cell, which we believe was responsible for the development of allergen-induced AHR. Thus, the development of AHR under different circumstances may require the presence of different forms or types of NKT cells, including type I as well as type II NKT cells. Each may be important in human patients with distinct forms of bronchial asthma.
Acknowledgments
We thank Mark Exley (Beth Israel Deaconess Medical Center, Harvard Medical School) for providing the hybridoma 3C11 mAb and CD1d−/− mice, Masaru Taniguchi and Toshinori Nakayama (Chiba University) for the Jα18−/− mice, the National Institutes of Health Tetramer Facility for CD1d tetramers, and Dr. Sam Strober (Stanford University) for critical discussions.
Footnotes
This work was supported by Grants HL062348 and AI26322 from the National Institutes of Health, funds from the Bunning Food Allergy Project, and a research fund from Chonnam National University Research Institute of Medical Sciences (to Y.I.K.).
Abbreviations used in this paper: AHR, airway hyperreactivity; α-GalCer, α-galactosylceramide; BAL, bronchoalveolar lavage; β2m, β2-microgolubin; DN, double negative; WT, wild type; iNKT, invariant NKT; RL, ling resistance.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat. Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 3.Lisbonne M, Diem S, de Castro Keller A, Lefort J, Araujo L, Hachem P, Fourneau J, Sidobre S, Kronenberg M, Taniguchi M, et al. Cutting edge: invariant Vα14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J. Immunol. 2003;171:1637–1641. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda J, Naidenko O, Gapin L, Nakayama T, Taniguchi M, Wang C, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human Vα24 natural killer T cells. J. Exp. Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang L, Adkins B, Deyev V, Podack ER. Essential role of TNF receptor superfamily 25 (TNFRSF25) in the development of allergic lung inflammation. J. Exp. Med. 2008;205:1037–1048. doi: 10.1084/jem.20072528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, Zhu M, Iwakura Y, Savage PB, Dekruyff RH, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J. Exp. Med. 2008;205:385–393. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, Benoit LA, Byers DE, Alevy Y, Tucker J, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat. Med. 2008;14:633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, DeKruyff RH, Umetsu DT. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N. Engl. J. Med. 2006;354:1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 10.Umetsu DT, DeKruyff RH. A role for natural killer T cells in asthma. Nat Rev Immunol. 2006;6:953–958. doi: 10.1038/nri1968. [DOI] [PubMed] [Google Scholar]
- 11.Baron JL, Gardiner L, Nishimura S, Shinkai K, Locksley R, Ganem D. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity. 2002;16:583–594. doi: 10.1016/s1074-7613(02)00305-9. [DOI] [PubMed] [Google Scholar]
- 12.Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ, Berzofsky JA. A nonclassical non-Vα4Jα18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J. Exp. Med. 2005;202:1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg RS, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J. Clin. Invest. 2004;113:1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown D, Fowell D, Corry D, Wynn T, Moskowitz N, Cheever A, Locksley R, Reiner S. β2-microglobulin-dependent NK1.1+ T cells are not essential for T helper cell 2 immune responses. J. Exp. Med. 1996;184:1295–1304. doi: 10.1084/jem.184.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Rogers KH, Lewis DB. β2-microglobulin-dependent T cells are dispensable for allergen-induced T helper 2 responses. J. Exp. Med. 1996;184:1507–1512. doi: 10.1084/jem.184.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc. Natl. Acad. Sci. USA. 2006;103:2782–2787. doi: 10.1073/pnas.0510282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattner J, DeBord KL, Ismail N, Goff RD, Cantu C, III, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 18.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J. Clin. Invest. 1999;103:175–183. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsitoura DC, DeKruyff RH, Lamb JR, Umetsu DT. Intranasal exposure to protein antigen induces immunological tolerance mediated by functionally disabled CD4+ T cells. J. Immunol. 1999;163:2592–2600. [PubMed] [Google Scholar]
- 20.Amano M, Baumgarth N, Dick MD, Brossay L, Kronenberg M, Herzenberg LA, Strober S. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: β2-microglobulin-dependent and independent forms. J. Immunol. 1998;161:1710–1717. [PubMed] [Google Scholar]
- 21.Bernabeu C, van de Rijn M, Lerch PG, Terhorst CP. β2-microglobulin from serum associates with MHC class I antigens on the surface of cultured cells. Nature. 1984;308:642–645. doi: 10.1038/308642a0. [DOI] [PubMed] [Google Scholar]
- 22.Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J. Exp. Med. 1995;182:993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balk SP, Burke S, Polischuk JE, Frantz ME, Yang L, Porcelli S, Colgan SP, Blumberg RS. β2-microglobulin-independent MHC class Ib molecule expressed by human intestinal epithelium. Science. 1994;265:259–262. doi: 10.1126/science.7517575. [DOI] [PubMed] [Google Scholar]
- 24.Kim HS, Garcia J, Exley M, Johnson KW, Balk SP, Blumberg RS. Biochemical characterization of CD1d expression in the absence of β2-microglobulin. J. Biol. Chem. 1999;274:9289–9295. doi: 10.1074/jbc.274.14.9289. [DOI] [PubMed] [Google Scholar]
- 25.Maeda M, Shadeo A, MacFadyen A, Takei F. CD1d-independent NKT cells in β2-microglobulin-deficient mice have hybrid phenotype and function of NK and T cells. J. Immunol. 2004;172:6115–6122. doi: 10.4049/jimmunol.172.10.6115. [DOI] [PubMed] [Google Scholar]
- 26.Murakami M, Paul WE. Age-dependent appearance of NK1.1+ T cells in the livers of β2-microglobulin knockout and SJL mice. J. Immunol. 1998;160:2649–2654. [PubMed] [Google Scholar]
- 27.Duarte N, Stenstrom M, Campino S, Bergman ML, Lundholm M, Holmberg D, Cardell SL. Prevention of diabetes in nonobese diabetic mice mediated by CD1d-restricted nonclassical NKT cells. J. Immunol. 2004;173:3112–3118. doi: 10.4049/jimmunol.173.5.3112. [DOI] [PubMed] [Google Scholar]
- 28.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson D, Carbone D, Paul W, Berzofsky J. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat. Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 29.Seino K, Taniguchi M. Functionally distinct NKT cell subsets and subtypes. J. Exp. Med. 2005;202:1623–1626. doi: 10.1084/jem.20051600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brusasco V, Crimi E, Pellegrino R. Airway hyperresponsiveness in asthma: not just a matter of airway inflammation. Thorax. 1998;53:992–998. doi: 10.1136/thx.53.11.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crimi E, Spanevello A, Neri M, Ind PW, Rossi GA, Brusasco V. Dissociation between airway inflammation and airway hyperresponsiveness in allergic asthma. Am. J. Respir. Crit. Care Med. 1998;157:4–9. doi: 10.1164/ajrccm.157.1.9703002. [DOI] [PubMed] [Google Scholar]
- 32.National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J. Allergy Clin. Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 33.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL-17-producing NK1.1neg iNKT cell population involved in airway neutrophilia. J. Exp. Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting edge: NKT cells constitutively express IL-23 receptor and RORγt and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J. Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker C, Checkel J, Cammisuli S, Leibson PJ, Gleich GJ. IL-5 production by NK cells contributes to eosinophil infiltration in a mouse model of allergic inflammation. J. Immunol. 1998;161:1962–1969. [PubMed] [Google Scholar]
- 36.Korsgren M, Persson CG, Sundler F, Bjerke T, Hansson T, Chambers BJ, Hong S, Van Kaer L, Ljunggren HG, Korsgren O. Natural killer cells determine development of allergen-induced eosinophilic airway inflammation in mice. J. Exp. Med. 1999;189:553–562. doi: 10.1084/jem.189.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]