Table 2.
Studies of the Mixed-ligand Conditions and Control Experimentsa
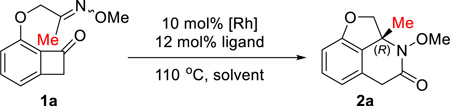 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst/Ligand | Additives | Solvent | Yieldb | er(R:S)c |
| 1 | [Rh(cod)(CH3CN)2]BF4/(R)-xyl-SDP | none | THF | 46% (63%) | 99.5:0.5 |
| 2 | [Rh(cod)(CH3CN)2]BF4/(R)-xyl-SDP | none | 1,4-dioxane | 51% (85%) | 99:1 |
| 3 | [Rh(cod)2]BF4/(R)-xyl-SDP | none | 1,4-dioxane | 53% (90%) | 99:1 |
| 4d | [Rh(cod)2]BF4/(R)-xyl-SDP | none | 1,4-dioxane | 55% (85%) | 99:1 |
| 5 | [Rh(cod)2]BF4/(S)-xyl-BINAP | none | 1,4-dioxane | 79% | 96:4 |
| 6 | [Rh(cod)2]BF4/(R)-xyl-SDP : (S)-xyl-BINAP = 1:1 | none | 1,4-dioxane | 63% | 97:3 |
| 7 | [Rh(cod)2]BF4/(R)-xyl-SDP : (R)-xyl-BINAP = 1:1 | none | 1,4-dioxane | 75% | 45:55 |
| 8 | [Rh(cod)2]BF4/(R)-xyl-SDP : (rac)-xyl-BINAP = 1:1 | none | 1,4-dioxane | 74% | 66:34 |
| 9d | [Rh(cod)2]BF4/(R)-xyl-SDP : (S)-xyl-BINAP = 1:1 | none | 1,4-dioxane | 72% | 97.2:2.5 |
| 10d | [Rh(cod)(CH3CN)2]BF4/(R)-xyl-SDP: (S)-xyl-BINAP = 1:1 | none | 1,4-dioxane | 61% | 97:3 |
| 11d | [Rh(cod)2]BF4/(R)-xyl-SDP : (S)-xyl-BINAP = 2:1 | none | 1,4-dioxane | 60% | 98.5:1.5 |
| 12d | [Rh(cod)2]BF4/(R)-xyl-SDP : (S)-xyl-BINAP = 5:1 | none | 1,4-dioxane | 54% | 99:1 |
| variations from entry 9 | |||||
| 13 | w/o Rh catalysts | none | 1,4-dioxane | 0% | N/A |
| 14 | w/o ligand | none | 1,4-dioxane | 0% | N/A |
| 15 | w/o catalyst & ligand | none | 1,4-dioxane | 0% | N/A |
| 16 | w/o catalyst & ligand | 20 mol% ZnCI2 | 1,4-dioxane | 0% | N/A |
| 17 | w/o catalyst & ligand | 20 mol% AICI3 | 1,4-dioxane | 0% | N/A |
| 18 | w/o catalyst & ligand | 20 mol% B(C6F5)3 | 1,4-dioxane | 0% | N/A |
Unless otherwise mentioned, the reaction was run with 10 mol % rhodium complex (based on the metal) and 12 mol % ligand on a 0.1 mmol scale at 110 °C for 48 h; numbers in parenthesis are yields based on recovered starting material (brsm).
Isolated yield.
Determined by chiral HPLC.
Rhodium complex (5 mol%) and mixed ligands (6 mol%) were added initially; the reaction mixture was stirred at 110 °C for 24 h before another portion of the same catalyst was added.
