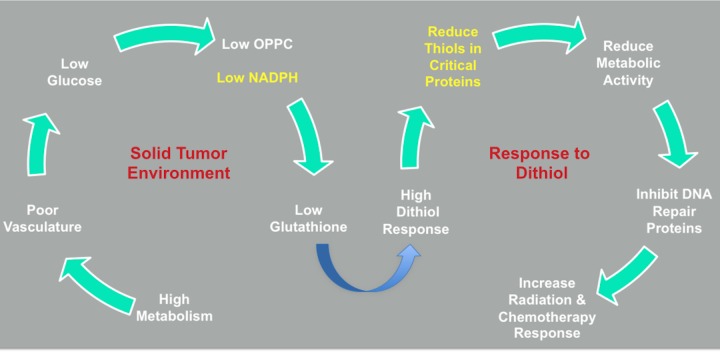
Figure 6. TTL-315 response model.
TTL-315 is proposed to interfere with thiol redox homeostasis and elicit cell death in glucose-deprived cells where its detoxification is inefficient. A detailed discussion of the bioreductive/detoxification pathways involved have been discussed elsewhere [10]. In the solid tumor microenvironment, the high glucose metabolism combined with a poor blood vasculature leads to glucose deprivation, low OPPC activity and reduced NADPH generation. This situation strains the levels of glutathione needed to preserve thiol homeostasis, which as a result becomes vulnerable to the additional stress created by dithiols that would be reduced by glutathione-dependent redox processes. By intensifying the competition for these processes, thiol redox control of critical cell proteins including DNA repair proteins is compromised. DNA-damaging chemotherapy and radiotherapy provide further strains that cooperate to heighten cellular demise. In attacking this weak point, TTL-315 as a dithiol stressor in glucose-deprived solid tumors may offer additional benefits to cancer management through production of its bioreductive product 2-mercaptopropionyl glycine (tiopronin), as discussed in the text.