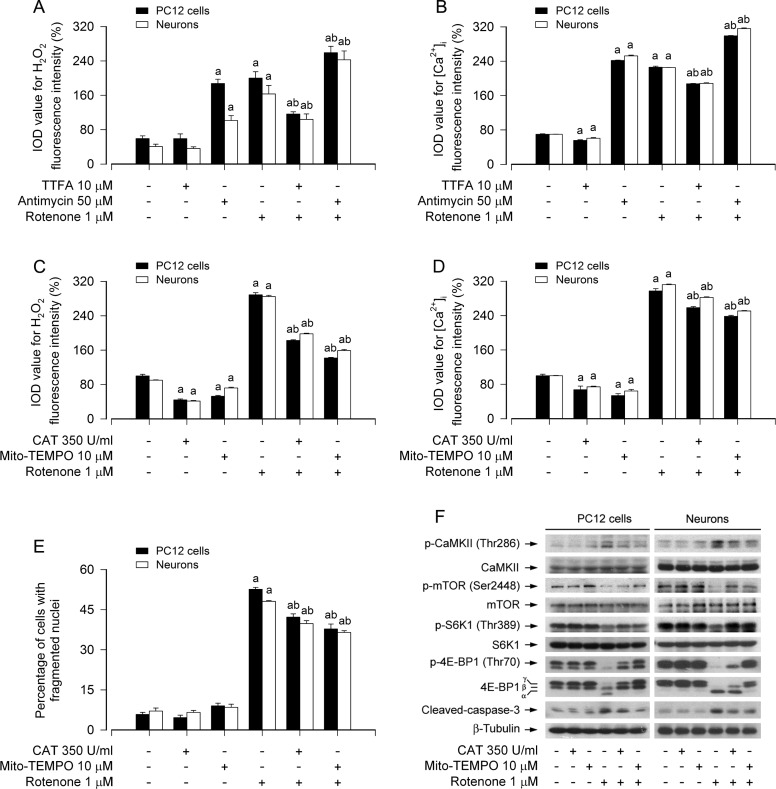
Figure 6. Rotenone induces mitochondrial H2O2-dependent [Ca2+]i elevation activating CaMKII, leading to inhibition of mTOR pathway and neuronal apoptosis.
PC12 cells and primary neurons were treated with rotenone (1 μM) in the presence or absence of TTFA (10 μM) or antimycin A (50 μM) for 24 h, or pretreated with/without CAT (350 U/ml) or Mito-TEMPO (10 μM) for 1 h and then exposed to rotenone (1 μM) for 24 h. Fluorescence intensity for cell H2O2 (A, C). and [Ca2+]i (B, D) was imaged and quantified using a peroxide-selective probe H2DCFDA and an intracellular Ca2+ indicator dye Fluo-3/AM, respectively. Cell apoptosis was assayed using DAPI staining (E). Total cell lysates were subjected to Western blotting using indicated antibodies (F). The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. A. There existed an obvious decline in H2O2 fluorescence during co-treatment with rotenone and TTFA in the cells, whereas a further increase in H2O2 level in the cells exposed to rotenone in the presence of antimycin A. B. Administration of TTFA elicited a significant reduction of [Ca2+]i, yet antimycin A resulted in an enhancement of [Ca2+]i triggered by rotenone. C.-E. Pretreatment with CAT or Mito-TEMPO significantly reduced intracellular H2O2 and [Ca2+]i levels (C, D) and prevented apoptosis in the cells induced by rotenone (E). F. Pretreatment with CAT or Mito-TEMPO substantially attenuated rotenone-induced activation of CaMKII phosphorylation, inhibition of mTOR, S6K1/4E-BP1 phosphorylation, and cleaved-caspase-3 in the cells. Results are presented as mean ± SE (n = 5). aP < 0.05, difference with control group; bP < 0.05, difference with 0.5 μM rotenone group; cP < 0.05, difference with 1 μM rotenone group.