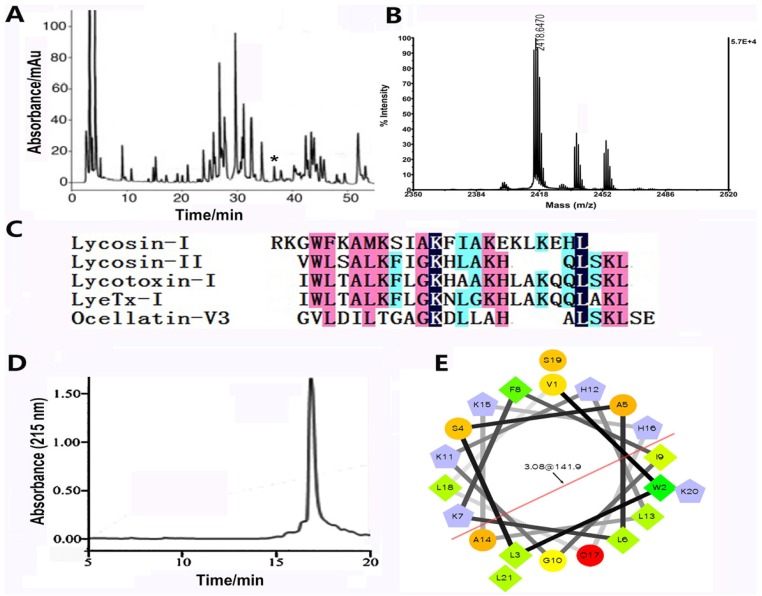
Figure 1.
Purification and characterization of lycosin-II. (A) Purification of lycosin-II by RP-HPLC (column, Vydac, C18, 300 Å, 4.6 mm× 250 mm). Venom components were eluted using a linear acetonitrile gradient (0%–60% acetonitrile/0.1% TFA in 60 min) at a flow rate of 1.0 ml/min. Elution of peptides was monitored at 215 nm. The peak labeled with an asterisk (*) contains lycosin-II. (B) MALDI-TOF MS of lycosin-II. (C) Multiple sequence alignment. Lycosin-II shows some similarity with some antimicrobial peptides. (D) Purification of synthetic lycosin-II by using RP-HPLC. (E) The α-helical wheel projection of lycosin-II, showing the amphipathic and cationic α-helix configuration of lycosin-II.