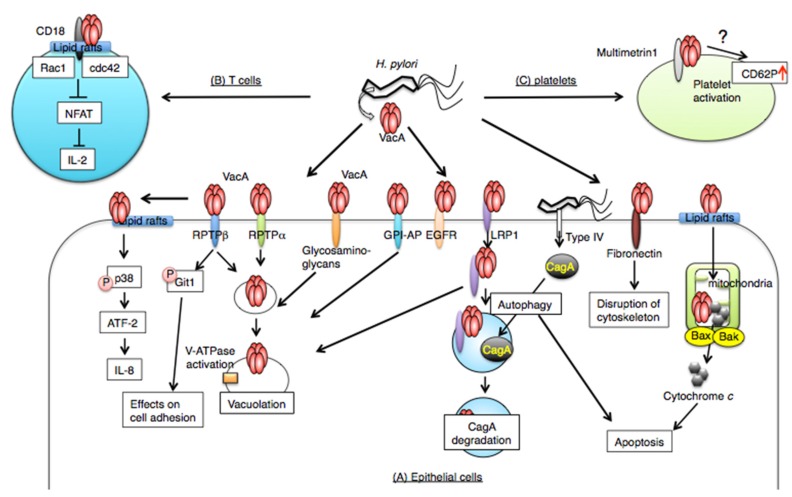
Figure 1.
VacA receptors. After VacA binding to receptors, it is translocated into cells by endocytosis and is responsible for multiple effects. (A) In epithelial cells, VacA binds to several receptors on target cells. Most of the receptors (e.g., RPTPα, RPTPβ, glycosaminoglycan, GPI-AP, EGFR, LRP1, fibronectin, sphingomyelin, lipid rafts) are involved in VacA uptake and vacuolating activity. In addition, lipid rafts are associated with VacA-induced apoptosis, which is also controlled by LRP1. VacA-induced apoptosis is caused by Bax/Bak conformational changes, leading to cytochrome c release. Interaction between VacA and LRP1 induces autophagy, which regulates stability of CagA released into cells by the H. pylori type IV secretion system. Signaling of VacA bound to fibronectin regulates cell adhesion and cytoskeletal organization. (B) On T cells, a β2 integrin (CD18) subunit interacts with VacA, leading to activation of cdc42 and Rac1, followed by VacA uptake. Intracellular, VacA impairs NFAT, leading to inhibition of IL-2 transcription. (C) Multimetrin 1 is a candidate for VacA receptors on platelets. Effects on VacA–multimetrin 1 interactions may be facilitated by CD62P expression on platelets.