Abstract
Studies of receptor-mediated lipoprotein metabolic pathways in avian species have revealed that physiological intricacies of specific cell types are highly analogous to those in mammals. A prime example for the power of comparative studies across different animal kingdoms, elucidated in the chicken, is that the expression of different lipoprotein receptors in somatic cells and oocytes are the key to oocyte growth. In avian species, yolk precursor transport from the hen's liver to rapidly growing oocytes and the subsequent transfer of yolk nutrients via the yolk sac to the developing embryo are highly efficient processes. Oocytes grow from a diameter of 5 mm to 2.5-3 cm in only 7 days, and the yolk sac transfers nutrients from the yolk stored in the mature oocyte to the embryo within just 2 weeks. The underlying key transport mechanism is receptor-mediated endocytosis of macromolecules, i.e., of hepatically synthesized yolk precursors for oocyte growth, and of mature yolk components for embryo nutrition, respectively. Recently, the receptors involved, as well as the role of lipoprotein synthesis in the yolk sac have been identified. As outlined here, lipoprotein degradation/resynthesis cycles and the expression of lipoprotein receptors are not only coordinated with the establishment of the follicular architecture embedding the oocyte, but also with the generation of the yolk sac vasculature essential for nutrient transfer to the embryo.
Keywords: chicken embryo, oocyte, yolk sac, lipoprotein receptors, vascularization
Chicken oocyte growth
Introduction
The most astounding events in the reproduction of oviparous (egg-laying) species are the growth and development of the female germ cell. The events leading to a mature oocyte in a wide variety of species are very similar[1]. Oocytes derive from mitotic cells called oogonia, which develop from primordial germ cells migrating into the ovary during early embryogenesis. After various periods of mitotic proliferation within the ovary, oogonia enter meiosis. The cells arrest in prophase of division I of meiosis when the chromosomes take on a “lampbrush” configuration (diplotene) characterized by high transcriptional activity. These meiotically arrested female germ cells, termed primary oocytes, enter growth periods whose length is species-dependent. During this growth, primary oocytes acquire all components of the machinery subsequently required for embryo development, including ribosomes, tRNAs, cytoplasmic organelles, mRNAs, and “early” yolk. In the female domesticated chicken (Gallus gallus domesticus) and likely in all birds, fertilization-competent oocytes develop continuously in follicles of the left hand ovary (the right hand ovary is obliterated during embryogenesis).
Chicken oocytes grow in three phases[2]: first, for several months, numerous microscopically small oocytes increase in size to 2-3 mm diameter, which still lack the typical ‘yellow’ yolk[3]; second, several of these oocytes continue to grow slowly, and third, at reaching a diameter of 5-6 mm, an estimated 75% of these oocytes are destined for atresia (i.e., resorption), and one the remaining oocytes enters the last, very rapid growth phase, reaches a diameter of ca. 30 mm, and ovulates. This final stage is characterized by a dramatic 7-day growth spurt during which the oocyte extracts from the circulation plasma-borne components amounting to up to 14 mL of yolk[4]. The yolk is comprised of lipid imported in the form of lipoproteins, mainly very low density lipoprotein (VLDL) and vitellogenin (VTG), which contribute ca. 5 g of triglycerides and 230 mg of cholesterol to the yolk mass. Lipoproteins and most of the additional minor yolk precursors are synthesized in and secreted from the liver and taken up by the growing oocytes via endocytotic processes involving specific cell-surface receptors. Apparently, the oocyte itself does not produce any yolk components. Ovulation of the largest oocyte occurs every 25 hour and triggers the beginnning of the rapid growth phase of the next oocyte in line (laid as egg 7-8 days later), establishing a size- and time-related hierarchy of preovulatory follicles. If the hen has been inseminated, fertilization of the ovulated oocyte occurs in the uppermost portion of the oviduct. During the next 25 hour of migration through the lumen of the oviduct, structural components stabilizing the oocyte proper, egg white proteins, water, egg membranes, and the shell are deposited around the oocyte to form the laid egg.
Although the yolk appears homogeneous, it actually is a rather complex and precisely compartmentalized structure[3-4]. From the germinal vesicle (the visible white spot on the oocyte surface), the latebra, a pear-shaped yolk-free cytoplasmic region, extends radially into the center of the oocyte. A medial cross-section reveals 7 concentric shells of yellow yolk (likely related to the 7 days of massive yolk deposition), which are separated by layers of oocytic cytoplasm that are connected to the latebra, thereby forming a continuous cytoplasmic compartment. The cytoskeletal elements and regulatory mechanisms that generate this structural organization, which may be unique to avian oocytes, are not known in any detail.
As will be described in this review, most of the contents of the oocyte are imported by the action of multifunctional oocyte-specific receptors belonging to the LDL receptor (LDLR) gene family. Both close and distant relatives of this family have been identified in the chicken, which thus has been established as a prime model to investigate the molecular genetics of these important receptors.
Lessons from the chicken: receptor-mediated oocyte growth
Molecular information on any of the proteins involved in oocyte growth and systemic lipoprotein transport in the laying hen was lacking until the mid-1980's, although the presence of bona-fide lipoprotein particles in yolk was highly suggestive of receptor-mediated processes for yolk lipoprotein deposition. Indeed, since then our studies on yolk precursor transport in the laying hen have not only provided proof for this concept, but also have revealed surprising new aspects of lipoprotein receptor biology with relevance to other species.
It is now clear that a 95-kDa protein in the plasma membrane of the oocyte binds both major yolk lipoproteins, VLDL and VTG[5]. This receptor protein reacts with antibodies to mammalian LDLRs and recognizes apolipoprotein (apo) E[6], an apo produced by mammals, but not by birds. These properties predicted that the oocyte receptor for VLDL and VTG is a homologue of mammalian LDL receptors which recognize apoB and apoE. The LDLR superfamily is defined by common structural elements with a high degree of sequence identity (70%-100%) between the molecules harbouring them, and in a wide range of species. Their conserved sequences likely have evolved from an ancestral gene by duplication and/or exon shuffling events. The most typical of these elements are the so-called LA repeats, which are tandemly arranged in clusters and form the ligand binding domains of LDLR family members. The classical LDLRs are characterized by the presence of seven LA repeats in their ligand binding domains, whereas the VLDLRs contain a cluster of eight LA repeats[7]. Molecular cloning of the 95-kDa oocyte protein indeed revealed an eight-repeat ligand binding domain[8] which was confirmed to bind apoE despite the fact that APOE is absent from the chicken genome. Thus, in order to distinguish this chicken oocyte receptor from mammalian VLDRs, we subsequently termed it LR8 (LDL receptor Relative with 8 LA repeats). The gene specifying LR8 is located on the galline sex chromosome Z[8-9]. As discussed below, in addition to the high evolutionary conservation of eight-repeat receptors, the absence in chicken oocyte LR8 of a serine- and threonine-rich domain carrying O-linked carbohydrate chains appears significant.
Important functional insights into VLDRs were gained from further studies in the chicken. Whereas the spectrum of physiological functions of mammalian VLDLRs is not yet fully delineated, the role of chicken LR8 is documented by both biochemical and genetic evidence[10]. LR8 mediates a key step in the reproductive effort of the hen, i.e., normal oocyte growth via yolk deposition. This conclusion can be drawn from the fact that functional absence of LR8 blocks oocytes from entering into the rapid growth phase. Consequently, the hens fail to lay eggs and to produce offspring. This phenotype is observed in a rare chicken strain carrying a single mutation at the VLDLR locus (on the sex chromosome Z, see above) termed ‘restricted ovulator’ (R/O) strain. Furthermore, as a consequence of the failure to deposit VLDL and VTG, which are produced at normal levels, into their oocytes, the mutant females develop severe hyperlipidemia and features of atherosclerosis[11]. Carrier roosters (genotype, VLDLR-/VLDLR) have normal lipid metabolism, as expected from our finding that LR8 is expressed almost exclusively in oocytes. Thus, -/VLDLR- females, which in fact represent a model for an oocyte-specific receptor defect leading to familial hypercholesterolemia, are sterile due to non-laying. In addition to VLDL and VTG[5], the receptor was shown to bind riboflavin-binding protein complexed with VTG[12] and clusterin (apolipoprotein J;[13]), components which fail to accumulate in the yolk of the atretic R/O oocytes.
The R/O allele of VLDLR carries a point mutation (G to C substitution) resulting in the replacement of cysteine-682 in the extracellular domain of LR8, located outside the binding domain, with a serine residue. Interestingly, the first ever delineated mutation in the human LDLR gene causing familial hypercholesterolemia occurred exactly in the equivalent position[14]. The disruption of a disulfide bond by the loss of the cysteine residue due to this mutation was shown to cause protein misfolding accompanied by rapid intracellular degradation of the altered receptor molecules[14]. In last consequence, the mutant LR8 protein does not reach the plasma membrane of oocytes of R/O hens, and thus is unable to mediate the uptake of serum-derived yolk precursor molecules.
Further investigations of the lipoprotein receptor system of hens revealed that those tissues which express the VLDLR in mammals, i.e., heart, skeletal muscle, brain, and adipose tissue, but not the liver, also express LR8 in the chicken, albeit at very low levels (approx. 0,5%) compared to the oocytes. Interestingly, the structures of the major VLDLR isoforms in mammals and the chicken LR8 differ by the presence (in mammals) and absence (in chicken oocytes) of the O-linked sugar domain (see above), respectively. The absence or presence of this domain of approximately 60 amino acids arises by differential splicing of the precursor mRNA. Thus, we performed detailed studies on the expression of LR8 splice variants in the chicken (for simplicity, the longer form is termed LR8+, and the shorter one, LR8-). It was shown that somatic cells and tissues, in particular granulosa cells, heart, and skeletal muscle express predominantly LR8+, while the oocyte express only LR8-[2].
These results in the laying hen demonstrate that the oocytic LR8- is a multifunctional receptor for the transport of lipoproteins and other components[7] required for embryonic growth. It is entirely possible that this holds true for the VLDLR in other tissues, including the mammalian ovary. LR8+, on the other hand, likely performs analogous functions in mammals and oviparous species, as they express this isoform in the same tissues. The molecular and functional properties of LR8 strengthen the hypothesis[6,15] that it is the product of an ancient gene that has retained the ability to interact with many ligands of younger LDLR superfamilymembers. In this context, VTG, absent from mammals, and apoE, not found in birds, have been suggested to be functional analogues, as they show common biochemical properties and regions of sequence similarities[6,15]. Thus, triglyceride-rich particles, the likely physiological substrate for mammalian VLDLRs, could be transported into metabolically active tissues (such as muscle, where receptors are abundant), while in avian oocytes the uptake of VTG, VLDL, and other hepatically synthesized yolk precursors by LR8 provides nutrients and energy for the developing embryo.
Nutrient transport by the chicken yolk sac
Introduction
The above described findings are relevant to one of the crucial requirements for successful reproduction, i.e., the generation of mature, fertilization-competent oocytes. Once the zygote has formed, embryo development starts. In the following section, the features of a mechanism assuring normal growth and development of embryos in egg-laying (oviparous) species is described. In general, normal development of the embryo depends on the adequate supply with nutrients from maternal sources. When the mammalian placenta has become established, it provides the maternal-fetal interface mediating nutrient transfer between the maternal and embryonic circulation. However, before the placental circulation becomes fully functional during neural tube closure, the mammalian so-called visceral yolk sac (YS) is essential in providing nutrition to the developing embryo. In oviparous species, such as the chicken, the functionally equivalent interface is provided by the YS, which encloses the yolk that originally has been taken up and stored by the oocyte, as described in section Chicken Oocyte Growth. The yolk, the almost exclusive source of nutrients for the developing embryo, contains macromolecular complexes comprising lipids, proteins, vitamins, minerals, and other essential micronutrients. At the onset of gastrulation[16], the formation of the mammalian visceral YS as well as the chicken YS is initiated; when completed the YSs are composed of cells derived from all three germ layers. Avian YS formation is a highly coordinated growth process in which rapidly dividing ectodermal cells spread from the embryo proper to ultimately cover the entire yolk compartment[17]. Trailing the migrating front of the ectodermal layer, and between the yolk surface and the ectoderm, the YS’s endodermal epithelial cells (EECs) proliferate and follow the ectodermal cells to form a tight epithelial layer, which remains in close contact with the yolk over the entire period of embryo development[17-18]. Finally, tightly associated with the basal aspect of the EECs, cells derived from the so-called splanchnic mesoderm migrate into the space between the ectodermal and endodermal layers and form a network of capillaries in tight contact with the neighboring two layers[19]. Thereby, the three germ layer cell types generate the fully functional YS, which is capable of mediating the targeted transfer of yolk-derived nutrient components to the embryonic circulation, as described below.
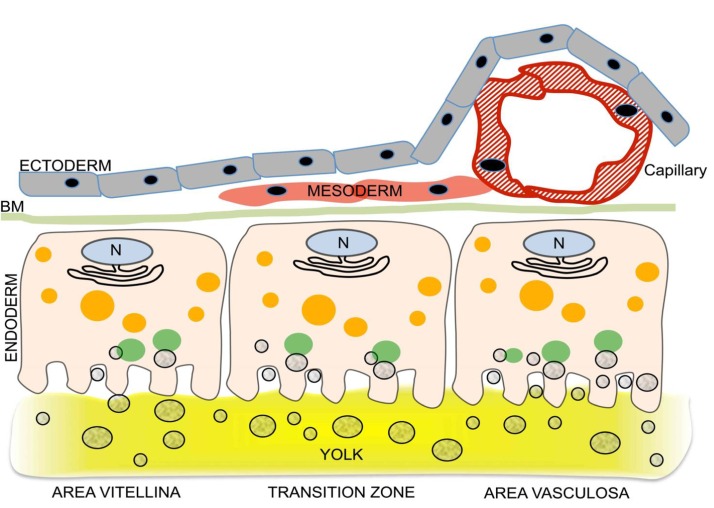
During much of its growth, the chicken YS consists of three morphologically and functionally different regions (for an overview, see Fig.1). These are termed (i) area pellucida, the central area of the early blastoderm which is considered part of the embryo proper; (ii) area vasculosa, which forms the major, vascularized part of the nascent YS; (iii) area vitellina, the YS's non-vascularized portion localized at the growth front[17,20]. Ultrastructural studies have provided information on the dynamics of the YS’s transformation during the phenotypic changes of the EECs upon transition of the area vitellina to the area vasculosa[17-18]. During this transition, the apical face of EECs acquires the typical characteristics of polarized epithelial cells with numerous villi, invaginations, and coated pits[17-18,20-21]. Taken together, these and other observations support the notion that the EECs in the area vasculosa are the most active yolk-absorbing cells in the entire YS[16,20,22].
Fig. 1. Acquisition of nutrient transport function by the developing chicken yolk sac (YS): differentiation of the endodermal epithelial cells (EECs) is linked to induction of vascularization.
Three regions of the growing YS (area pellucida not shown) can be distinguished, each schematically represented by one EEC and associated cells. The 3 regions are the area vitellina (left), a transition zone (center), and the area vasculosa (right). The area vitellina EECs lack endocytic receptors and nonspecifically phagocytose yolk components while they migrate along the yolk surface underneath the ectoderm. Due to the presence of Plin2 on the area vitellina LD surface (red circle around LDs in area vitellina), the lipids of the LDs, particularly triglycerides, are not available for extensive lipidation of ApoA-I, and therefore the predominant secreted lipoprotein particles have a high density (HDL). In the transition zone, characterized by migration of mesenchymal progenitor cells into the interstitium between ecto- and endoderm, receptors begin to be produced, phagocytotic yolk uptake continues, and as Plin2 levels drop, some LD lipids become available for increased lipoprotein synthesis and secretion. In the area vasculosa, blood vessel formation is coordinated with the enhanced production and localization of endocytic receptors with specificity for selected yolk components to the apical aspect of EECs, the disappearance of Plin2, and the expression of genes specifying MTP and additional apolipoproteins. The lipids liberated by lipolysis from LDs, and components derived from uptake and lysosomal processing of lipoproteins such as yVLDL and other yolk macromolecules are utilized for the assembly of lipoproteins containing newly synthesized ApoA-I, ApoB, and ApoA-V, i.e., VLDL-like and HDL-like particles which become efficiently secreted for transfer into the adjacent blood vessels, thereby supplying the embryo with nutrients generated by transformation of oocytic yolk in the EECs (adapted from Bauer et al.[36]).
Yolk sac development and nutrient transport to the embryo
While morphological aspects of differentiation of YS cells and the acquisition of functional features in the course of YS maturation have been well characterized, very little is known about the components and regulation of the mechanisms that accomplish the complex process of nutrient uptake from yolk into the EECs and subsequent transfer to the embryo. Previous gene expression profiling of area vasculosa EECs from 2-4 d old chick embryos revealed the enrichment of several transcripts for enzymes likely involved in lipid metabolism and for proteins possibly important for embryonic growth in these cells[22]. However, candidate genes whose products may be functionally involved in the uptake of yolk nutrients did not emerge from this analysis[21]. Therefore, we have used the growing chicken YS to identify the processes and molecules that provide it with the ability to transfer nutrients from the yolk to the embryo. The rationale for these investigations results from the questions (i) whether the mature chicken YS resembles the mammalian visceral YS, a tissue essential to embryo nutrition prior to the onset of placental circulation, and (ii) at which stage of YS development the tissue becomes competent for nutrient transport. To answer these questions, the characteristic phenotypic differentiation of EECs during the area vitellina/area vasculosa transition, which accompanies the YS’s vascularization, was exploited to identify and characterize proteins involved in the uptake of lipoprotein particles and other nutrient precursors from the yolk into the EECs. We showed that, subsequent to uptake and degradation of lipoproteins and other macromolecules from the yolk, the EECs synthesize lipoproteins de-novo that differ in composition from those in yolk and secrete them for targeting to the embryonic circulation. Importantly, the expression patterns of key molecules for nutrient transfer, such as endocytic receptor complexes, apolipoproteins, and lipid droplet-associated proteins, differ between EECs of the area vasculosa and the area vitellina. One of the most significant observations was that Plin2 (also known as ADRP) is found in the area vitellina, but not vasculosa. Plin2 has been shown to reduce the lipid droplet association of adipose triglyceride lipase and to slow triacylglycerol turnover (see Conclusion and Perspective). Thus, the findings support a model (Fig.1) in which the vascularized portion of the YS is its functionally active region, whereas the area vitellina, a tissue comparable with the extraembryonic endoderm in early rodent embryos[10,23] appears to have primarily lipid storage functions.
The specific genes and their products involved in nutrient transfer by the YS were identified in stepwise fashion. First, since the epithelium of the mature mammalian visceral YS expresses certain genes that have hitherto not been studied in the chicken, we established that these and other key components are indeed expressed in the fully developed avian YS. Importantly, the multiligand receptors cubilin and LDLR related protein 2 (LRP2 or megalin), as well as amnionless, which together form a trimeric endocytosis-competent complex[24,25], are produced only by the EEC layer of the area vasculosa, and not area vitellina. This receptor triad has been established as essential for nutrient transport by the mammalian visceral YS[26,27]. Mutations in any of the three genes or blocking their function by antibodies compromise normal embryonic development in rodents, especially affecting neural tube and brain formation[28,29]. Cubilin and LRP2 recognize a vast number of diverse ligands, many of which are yolk constituents, such as protein-bound vitamins, hormones, and a range of lipoproteins[30]. Furthermore, we have previously reported that the YS’s EECs also express LR8, which is capable of internalizing ApoB-containing lipoproteins such as VLDL, as well as VTG and clusterin[31], thus expanding the list of receptors involved in the uptake of yolk precursor macromolecules into the EECs. Similar to receptor expression, only the area vasculosa EECs produce MTP and secrete ApoA-I, ApoB, and ApoA-V as protein moieties of newly synthesized lipoproteins targeted for ultimate uptake and utilization by the embryo. In contrast, the EECs from the area vitellina produce almost exclusively HDL-like, ApoA-I-containing lipoprotein particles.
Conclusion and perspective
Taken together, these data support a model for differentiation of the developing chicken YS as a result of coordination of vascularization and acquisition of function, as outlined in Fig. 1. A key feature of this process at the molecular level is the switch of the EECs from a storage type to a metabolically highly active type of cells in synchrony with the acquisition of vasculature. This conversion is characterized by the onset of increased production of endocytic yolk protein receptors, accessory proteins, and apolipoproteins in addition to ApoA-I, as well as by the secretion of lipoproteins by EECs in the neo-vascularized region of the growing YS. Furthermore, in contrast to the EECs of the area vasculosa, which synthesize and secrete (apo)lipoproteins at high rates, the requirement for lipids stored in lipid droplets (LDs) in the EECs of the area vitellina (which produce low levels of lipoproteins) is expected to be low. This important difference might be regulated by proteins that play a role in the stability of LDs, i.e., via reducing the susceptibility of LDs to lipolysis and thus availability of substrate for lipoprotein synthesis. One of the known proteins that promotes the accumulation and growth of LDs in cells by compromising lipolysis is Plin2[32,33] (Fig.1). Indeed, Plin2 knock-out mice show elevated VLDL secretion rates and a decrease in hepatic lipid content[34], and knockdown of Plin2 in McA-RH7777 hepatoma cells results in increased VLDL secretion[35]. The absence of Plin2 from the area vasculosa EECs, but its high levels in the area vitellina, where lipoproteins containing ApoB are hardly produced at all, indicates that Plin2 performs a so far unknown regulatory function in lipid metabolism of the developing chicken YS. These results support the notion that a crosstalk between the processes of EEC differentiation and of vasculogenesis is essential to YS function and thus, for embryo development.
Acknowledgement
Original research in the author's laboratory was supported by Research Grants from the Austrian Science Fund, the Austrian National Bank and the Herzfelder Family Endowment.
References
- [1].Wassarman PM, Josefowicz WJ. Oocyte development in the mouse: an ultrastructural comparison of oocytes isolated at various stages of growth and meiotic competence[J]. J Morphol, 1978,156(2):209-235. [DOI] [PubMed] [Google Scholar]
- [2].Bujo H, Lindstedt KA, Hermann M, et al. Chicken oocytes and somatic cells express different splice variants of a multifunctional receptor[J]. J Biol Chem, 1995,270(40):23546-23551. [DOI] [PubMed] [Google Scholar]
- [3].Schjeide OA, Galey F, Grellert EA, et al. Macromolecules in oocyte maturation[J]. Biol Reprod, 1970,2:Suppl 2:14-43. [DOI] [PubMed] [Google Scholar]
- [4].Perry MM, Gilbert AB, Evans AJ. Electron microscope observations on the ovarian follicle of the domestic fowl during the rapid growth phase[J]. J Anat, 1978,125(Pt 3):481-497. [PMC free article] [PubMed] [Google Scholar]
- [5].Stifani S, Barber DL, Nimpf J, et al. A single chicken oocyte plasma membrane protein mediates uptake of very low density lipoprotein and vitellogenin[J]. Proc Natl Acad Sci U S A, 1990,87(5):1955-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Steyrer E, Barber DL, Schneider WJ. Evolution of lipoprotein receptors. The chicken oocyte receptor for very low density lipoprotein and vitellogenin binds the mammalian ligand apolipoprotein E[J]. J Biol Chem, 1990,265(32):19575-19581. [PubMed] [Google Scholar]
- [7].Schneider WJ, Osanger A, Waclawek M, et al. Oocyte growth in the chicken: receptors and more[J]. Biol Chem, 1998,379(8-9):965-971. [PubMed] [Google Scholar]
- [8].Bujo H, Hermann M, Kaderli MO, et al. Chicken oocyte growth is mediated by an eight ligand binding repeat member of the LDL receptor family[J]. EMBO J, 1994,13(21):5165-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nanda I, Zend-Ajusch E, Shan Z, et al. Conserved synteny between the chicken Z sex chromosome and human chromosome 9 includes the male regulatory gene DMRT1: a comparative (re)view on avian sex determination[J]. Cytogenet Cell Genet, 2000,89(1-2):67-78. [DOI] [PubMed] [Google Scholar]
- [10].Dunn NR, Hogan BL. How does the mouse get its trunk[J]? Nat Genet, 2001,27(4):351-352. [DOI] [PubMed] [Google Scholar]
- [11].Bujo H, Yamamoto T, Hayashi K, et al. Mutant oocytic low density lipoprotein receptor gene family member causes atherosclerosis and female sterility[J]. Proc Natl Acad Sci U S A, 1995,92(21):9905-9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mac Lachlan I, Nimp fJ, Schneider WJ. Avian riboflavin binding protein binds to lipoprotein receptors in association with vitellogenin[J]. J Biol Chem, 1994,269(39):24127-24132. [PubMed] [Google Scholar]
- [13].Mahon MG, Lindstedt KA, Hermann M, et al. Multiple involvement of clusterin in chicken ovarian follicle development. Binding to two oocyte-specific members of the low density lipoprotein receptor gene family[J]. J Biol Chem, 1999,274(7):4036-4044. [DOI] [PubMed] [Google Scholar]
- [14].Leitersdorf E, Tobin EJ, Davignon J, et al. Common low-density lipoprotein receptor mutations in the French Canadian population[J]. J Clin Invest, 1990,85(4):1014-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bujo H, Hermann M, Schneider WJ, et al. A new branch of the LDL-receptor family tree: VLDLreceptors[J]. Z Gastroenterol, 1996,34(Suppl 3):124-126. [PubMed] [Google Scholar]
- [16].Palis J, McGrath KE, Kingsley PD. Initiation of hematopoiesis and vasculogenesis in murine yolk sac explants[J]. Blood, 1995,86(1):156-163. [PubMed] [Google Scholar]
- [17].Yoshizaki N, Soga M, Ito Y, et al. Two-step consumption of yolk granules during the development of quail embryos[J]. Dev Growth Differ, 2004,46(3):229-238. [DOI] [PubMed] [Google Scholar]
- [18].Mobbs IG, McMillan DB. Transport across endodermal cells of the chick yolk sac during early stages of development[J]. Am J Anat, 1981,160(3):285-308. [DOI] [PubMed] [Google Scholar]
- [19].Sheng G. Primitive and definitive erythropoiesis in the yolk sac: a bird's eye view[J]. Int J Dev Biol, 2010,54(6-7):1033-1043. [DOI] [PubMed] [Google Scholar]
- [20].Mobbs IG, McMillan DB. Structure of the endodermal epithelium of the chick yolk sac during early stages of development[J]. Am J Anat, 1979,155(3):287-309. [DOI] [PubMed] [Google Scholar]
- [21].Nakazawa F, Alev C, Jakt LM, et al. Yolk sac endoderm is the major source of serum proteins and lipids and is involved in the regulation of vascular integrity in early chick development[J]. Dev Dyn, 2011,240(8):2002-2010. [DOI] [PubMed] [Google Scholar]
- [22].Sheng G, Foley AC. Diversification and conservation of the extraembryonic tissues in mediating nutrient uptake during amniote development[J]. Ann N Y Acad Sci, 2012,1271:97-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kwon GS, Fraser ST, Eakin GS, et al. Tg(Afp-GFP) expression marks primitive and definitive endoderm lineages during mouse development[J]. Dev Dyn, 2006,235(9):2549-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ahuja R, Yammani R, Bauer JA, et al. Interactions of cubilin with megalin and the product of the amnionless gene (AMN): effect on its stability[J]. Biochem J, 2008,410(2):301-308. [DOI] [PubMed] [Google Scholar]
- [25].Coudroy G, Gburek J, Kozyraki R, et al. Contribution of cubilin and amnionless to processing and membrane targeting of cubilin-amnionless complex[J]. J Am Soc Nephrol, 2005,16(8):2330-2337. [DOI] [PubMed] [Google Scholar]
- [26].Assemat E, Vinot S, Gofflot F, et al. Expression and role of cubilin in the internalization of nutrients during the peri-implantation development of the rodent embryo[J]. Biol Reprod, 2005,72(5):1079-1086. [DOI] [PubMed] [Google Scholar]
- [27].Zohn IE, Sarkar AA. The visceral yolk sac endoderm provides for absorption of nutrients to the embryo during neurulation[J]. Birth Defects Res A Clin Mol Teratol, 2010,88(8):593-600. [DOI] [PubMed] [Google Scholar]
- [28].Fisher CE, Howie SE. The role of megalin (LRP-2/Gp330) during development[J]. Dev Biol, 2006,296(2):279-297. [DOI] [PubMed] [Google Scholar]
- [29].Willnow TE, Hilpert J, Armstrong SA, et al. Defective forebrain development in mice lacking gp330/megalin[J]. Proc Natl Acad Sci U S A, 1996,93(16):8460-8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kozyraki R, Gofflot F. Multiligand endocytosis and congenital defects: roles of cubilin, megalin and amnionless[J]. Curr Pharm Des, 2007,13(29):3038-3046. [DOI] [PubMed] [Google Scholar]
- [31].Hermann M, Mahon MG, Lindstedt KA, et al. Lipoprotein receptors in extraembryonic tissues of the chicken[J]. J Biol Chem, 2000,275(22):16837-16844. [DOI] [PubMed] [Google Scholar]
- [32].Imamura M, Inoguchi T, Ikuyama S, et al. ADRP stimulates lipid accumulation and lipid droplet formation in murine fibroblasts[J]. Am J Physiol Endocrinol Metab, 2002,283(4):E775-783. [DOI] [PubMed] [Google Scholar]
- [33].Listenberger LL, Ostermeyer-Fay AG, Goldberg EB, et al. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover[J]. J Lipid Res, 2007,48(12):2751-2761. [DOI] [PubMed] [Google Scholar]
- [34].Chang BH, Li L, Saha P, et al. Absence of adipose differentiation related protein upregulates hepatic VLDL secretion, relieves hepatosteatosis, and improves whole body insulin resistance in leptin-deficient mice[J]. J Lipid Res, 2010,51(8):2132-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Magnusson B, Asp L, Boström P, et al. Adipocyte differentiation-related protein promotes fatty acid storage in cytosolic triglycerides, inhibits secretion of very low-density lipoproteins[J]. Arterioscler Thromb Vasc Biol, 2006,26(7):1566-1571. [DOI] [PubMed] [Google Scholar]
- [36].Bauer R, Plieschnig JA, Finkes T, et al. The developing chicken yolk sac acquires nutrient transport competence by an orchestrated differentiation process of its endodermal epithelial cells[J]. J Biol Chem, 2013,288(2):1088-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]