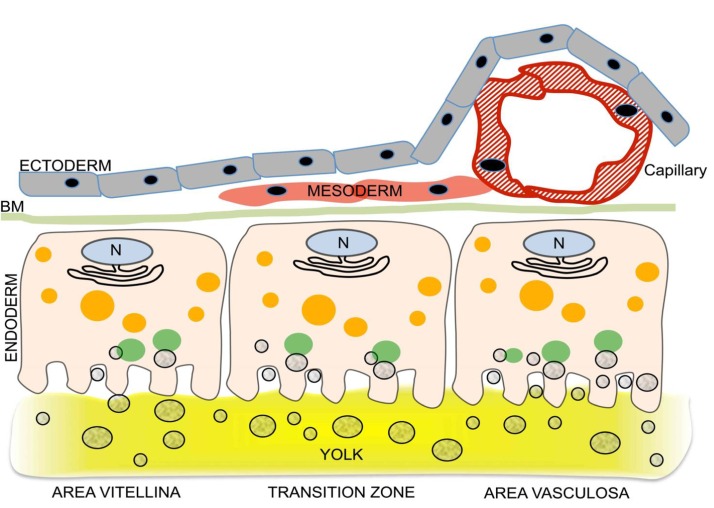
Fig. 1. Acquisition of nutrient transport function by the developing chicken yolk sac (YS): differentiation of the endodermal epithelial cells (EECs) is linked to induction of vascularization.
Three regions of the growing YS (area pellucida not shown) can be distinguished, each schematically represented by one EEC and associated cells. The 3 regions are the area vitellina (left), a transition zone (center), and the area vasculosa (right). The area vitellina EECs lack endocytic receptors and nonspecifically phagocytose yolk components while they migrate along the yolk surface underneath the ectoderm. Due to the presence of Plin2 on the area vitellina LD surface (red circle around LDs in area vitellina), the lipids of the LDs, particularly triglycerides, are not available for extensive lipidation of ApoA-I, and therefore the predominant secreted lipoprotein particles have a high density (HDL). In the transition zone, characterized by migration of mesenchymal progenitor cells into the interstitium between ecto- and endoderm, receptors begin to be produced, phagocytotic yolk uptake continues, and as Plin2 levels drop, some LD lipids become available for increased lipoprotein synthesis and secretion. In the area vasculosa, blood vessel formation is coordinated with the enhanced production and localization of endocytic receptors with specificity for selected yolk components to the apical aspect of EECs, the disappearance of Plin2, and the expression of genes specifying MTP and additional apolipoproteins. The lipids liberated by lipolysis from LDs, and components derived from uptake and lysosomal processing of lipoproteins such as yVLDL and other yolk macromolecules are utilized for the assembly of lipoproteins containing newly synthesized ApoA-I, ApoB, and ApoA-V, i.e., VLDL-like and HDL-like particles which become efficiently secreted for transfer into the adjacent blood vessels, thereby supplying the embryo with nutrients generated by transformation of oocytic yolk in the EECs (adapted from Bauer et al.[36]).