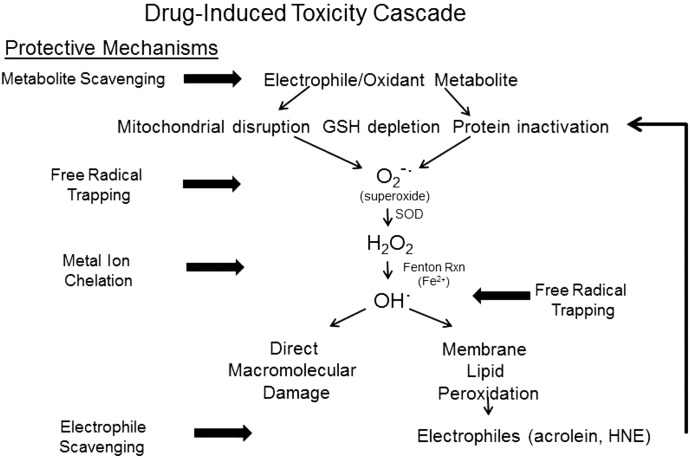
Fig. 10.
This figure presents a schematic diagram of a typical drug-induced toxicity pathway mediated by a soft electrophile metabolite. The illustration also highlights the possible molecular sites of intervention by cytoprotective enolate-forming compounds. As per our current understanding of drug-induced toxicities, the electrophilic metabolite (e.g., NAPQI) will form adducts with nucleophilic cysteine residues on GSH and proteins. Some of these electrophilic metabolites also function as oxidants. The resulting GSH depletion, protein inactivation, and mitochondrial disruption promote cellular oxidative stress by initiating the generation of superoxide anion (O2−·) and hydrogen peroxide (H2O2). Through metal-catalyzed Fenton reactions, these free radicals generate highly reactive hydroxyl radicals (OH·) that can cause direct macromolecular damage. In addition, the hydroxyl and superoxide radicals can initiate peroxidation of polyunsaturated fatty acids to yield α,β-unsaturated aldehydes (e.g., acrolein, 4-hydroxy-2-nonenal). As soft electrophiles, these aldehyde toxicants contribute to the cellular electrophile burden and can thereby augment cytotoxicity. Enolate-forming cytoprotectants such as THA can act at several steps in this cascade to truncate development of toxicity. As soft nucleophiles, these compounds can scavenge the initiating metabolite and can bind membrane lipid-derived aldehyde electrophiles. The ability of these chemicals to chelate transition metal ions can inhibit the Fenton reaction and subsequent generation of free radicals. The radicals that nonetheless develop can be trapped by aromatic enolate-forming compounds (e.g., THA, PG). Therefore, the multifunctional enol cytoprotectants represent a platform for development of effective compounds that can treat drug-induced toxicities and pathogenic conditions involving cellular oxidative stress. HNE, 4-hydroxy-2-nonenal; RXN, reaction.