TABLE 1.
Line structures and physicochemical characteristics of selected nucleophiles
| Compounda |
Structure (Ionization) |
Nucleophile |
ω−b,c |
σb |
pKa |
|---|---|---|---|---|---|
| 2-ACP (partial) | 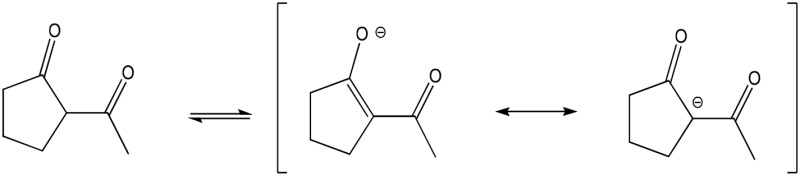 |
Enolate | 485 (204) | 418 | 7.8 |
| Phloretin (toxic) | 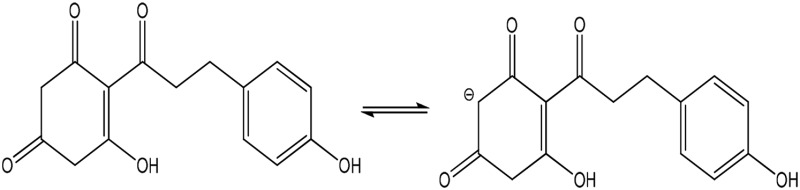 |
Enolate | 221 (105) | 494 | 7.3 |
| PG (full) | 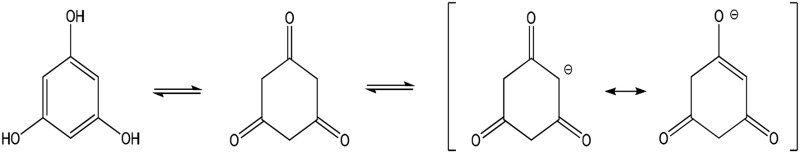 |
Enolate | 366 (133) | 540 | 8.5 |
| THA (full) | 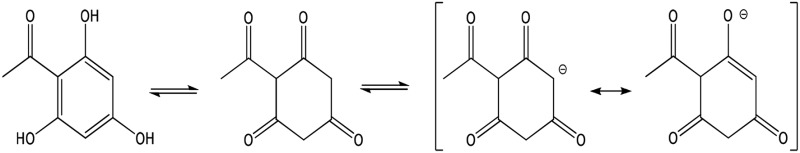 |
Enolate | 325 (114) | 485 | 7.7 |
| NAC (partial) | 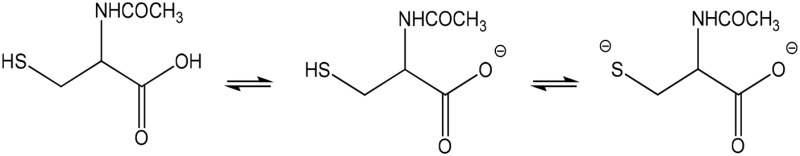 |
Thiolate | 667 (316) | 367 | 9.5 |
| GSH (partial) | 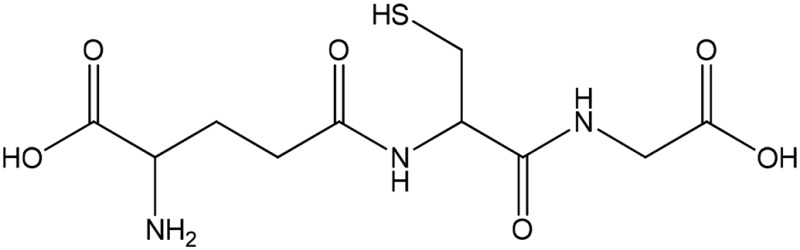 |
Thiolate | 548 (239) | 427 | 8.7 |
| N-acetyl-l-lysine (none) | 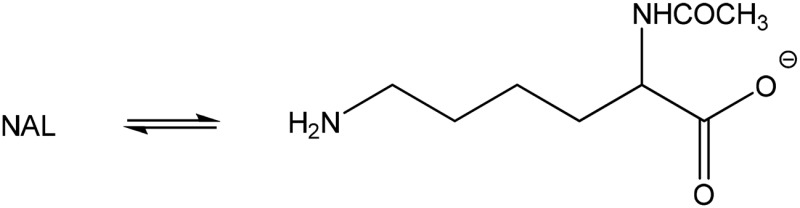 |
Amine | 387 (162) | 292 | 10 |
| Carnosine (none) | 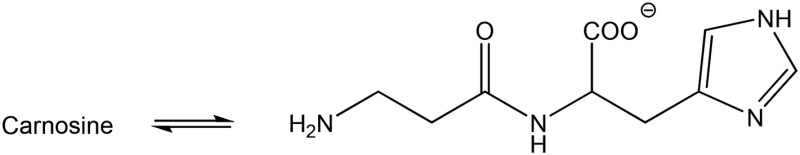 |
Amine | 389 (155) | 307 | 9.5 |
Data in parentdeses indicate level of cytoprotection.
Values are ×10−3 eV.
Values for reaction witd NAPQI (values in parentdeses are for reaction witd acrolein).
