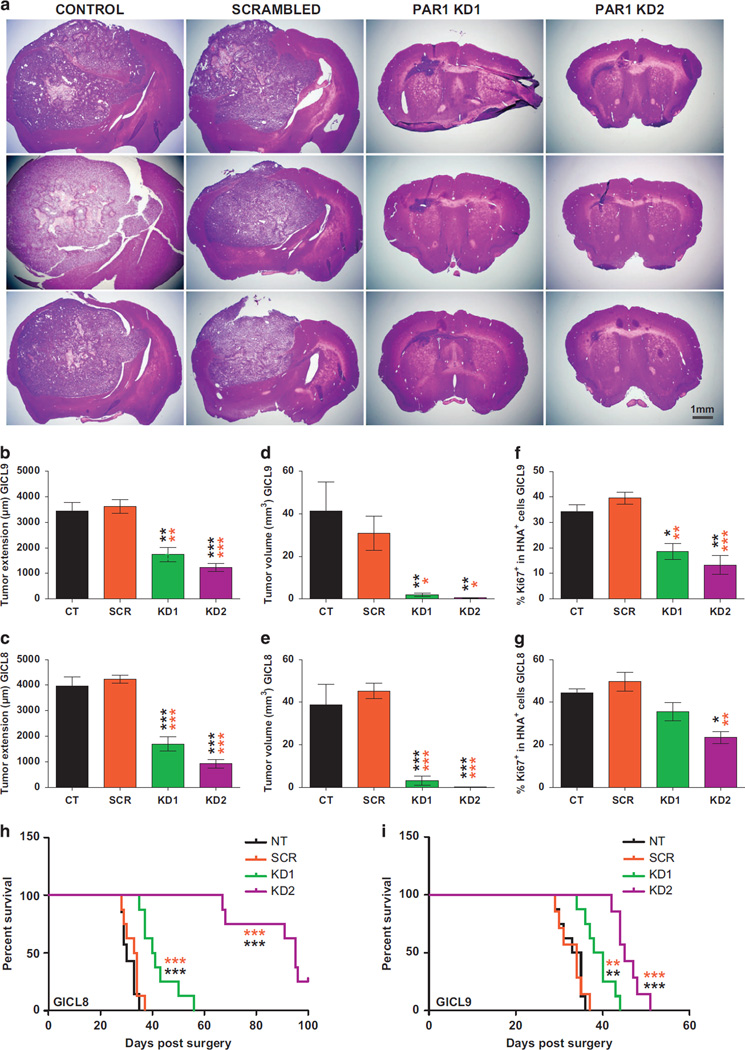
Figure 5.
PAR1 silencing suppresses the in vivo growth of TPC-derived tumors and prolongs survival Effects of PAR1 silencing on the in vivo expansion of A2B5+ GICLs derived from two different GBM (28 000 cells per animal, n = 5–6 mice per group), 4 weeks after transduction with PAR1 knockdown (KD) lentiviruses, compared with scrambled lentivirus (SCR) and control (CT) untransduced cells. (a) Hematoxylin-eosin stained sections of xenografts following intracranial implantation of A2B5+ TPCs. (b–g) Graphs representing the stereological analysis of the tumor extension, measured along the antero-posterior axis of xenograft mice brain (b, c); tumor volume (d, e); and proliferation of glioma TPCs as shown by the number of xenografted cells stained with the anti-human nuclei antigen (HNA) co-expressing the mitotic marker Ki67 (f, g), demonstrating a prominent inhibitory effect of PAR1 silencing on the tumorigenicity and mitotic activity of glioma A2B5+ TPCs relative to SCR and CT cells. Means ± s.e.m. P-values calculated using one-way ANOVA (P < 0.0001) followed by Tukey post-hoc comparisons with *P < 0.05; **P < 0.01; ***P < 0.001. Post-hoc comparisons between cells transduced with PAR1 KD shRNAi, compared with those transduced with SCR shRNAi untransduced CT cells are illustrated by orange and black stars, respectively. (h–i) Kaplan–Meier curves show an increase in median survival of mice bearing intracranial glioma TPCs transduced with PAR1 shRNA relative to SCR and CT mice. Log-rank analysis, (P < 0.0001) followed by pairwise comparison between all groups with **P < 0.01 and ***P < 0.001.