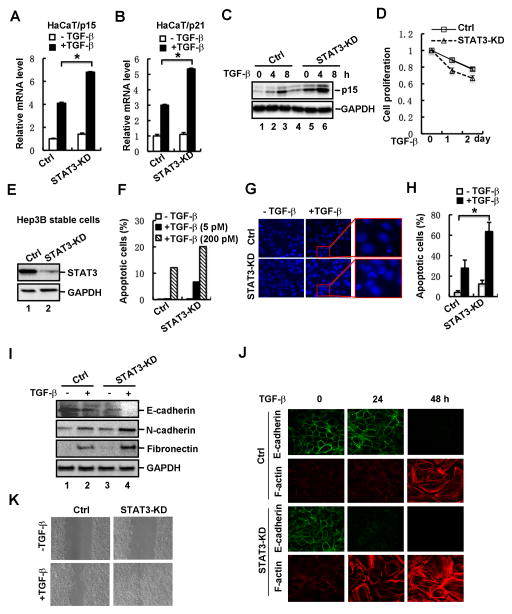
Fig. 3. Loss of STAT3 enhances TGF-β-induced physiological responses.
(A) Depletion of STAT3 increases TGF-β-induced p15 mRNA expression in HaCaT cells. Cells were treated with or without TGF-β (2 ng/ml) for 2 h, and total RNA was extracted for qRT-PCR analysis.
(B) Depletion of STAT3 increases TGF-β-induced p21 mRNA expression in HaCaT cells.
(C) Depletion of STAT3 enhances TGF-β-induced p15 protein expression in HaCaT cells. Cells were treated with TGF-β for 0, 4 and 8 h, respectively, and then the protein level of p15 was detected by using Western blotting.
(D) Depletion of STAT3 in HaCaT cells enhances TGF-β-induced growth arrest. STAT3 knockdown stable cells and control cells were treated with TGF-β, and cell growth rates were examined by actual cell numbers being counted at indicated time point (0, 1 and 2 d) of TGF-β treatment (1 ng/ml).
(E) Stable knockdown of STAT3 in Hep3B cells. The protein level of STAT3 was detected by using Western blotting.
(F) Depletion of STAT3 enhances TGF-β-induced cell apoptosis. STAT3 knockdown stable cells and control cells were treated with TGF-β at indicated concentrations. Apoptotic cells were analyzed by flow cytometry. STAT3 knockdown stable cells and control cells were treated with or without TGF-β (2 ng/ml) for 48 h.
(G) Apoptotic nuclei and DNA fragments were visualized by nuclei staining.
(H) Quantification of apoptotic cells in panel G.
(I) Depletion of STAT3 enhances TGF-β-induced EMT marker expression. STAT3 knockdown stable cells and control cells were treated with or without TGF-β (2 ng/ml) for 36 h, and the protein levels of E-cadherin, N-cadherin and Fibronectin were detected by Western blotting.
(J) Depletion of STAT3 enhances TGF-β-mediated EMT. STAT3 knockdown stable cells and control cells were treated with TGF-β (2 ng/ml) for 0, 24 and 48 h, respectively. Cells were fixed and immuno-stained with anti-E-cadherin or anti-F-Actin antibody, and imaged by confocal microscope.
(K) Depletion of STAT3 promotes cell migration. STAT3 knockdown stable cells and control cells were grown to confluence, followed by a scratch on the cell patch. The status of cell gap closure was recorded at 24 hours post-wound in the presence or absence of TGF-β (2 ng/ml).