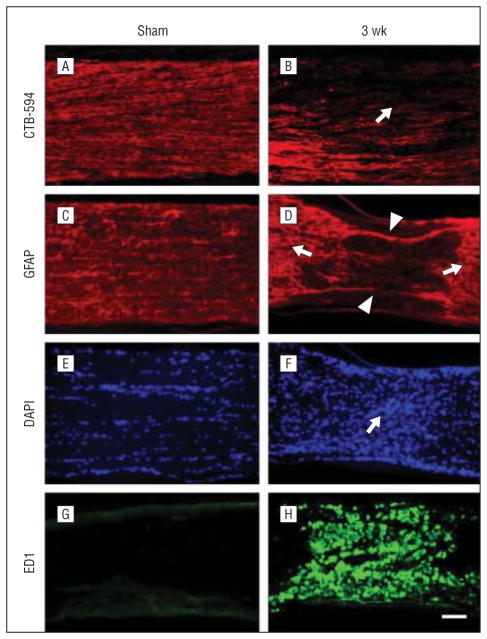
Figure 5.
Axon degeneration and glia activation after posterior ischemic optic neuropathy (PION). Longitudinal sections of optic nerve labeled for CTB-594 (axons), glial fibrillary acidic protein (GFAP; astrocyte processes), 4′6-diamidino-2-phenylindole (DAPI; nuclear), and ED1 (microglia/macrophages) in the sham-treated and PION-induced optic nerve. Anterograde labeling of retinal ganglion cell axons with CTB-594 (A and B). Axonal fluorescence appears dense and uniform in sham-operated animals (A, laser only/no erythrosin B). The fluorescence becomes much weaker in the lesioned area of the optic nerve at 3 weeks after PION induction (B, arrow). In the sham-treated optic nerve (laser only/no erythrosin B), astrocytic processes are transversely arranged (C); 3 weeks after PION, there was a loss of processes in the lesioned area (D, arrowheads), with adjacent astrocytic hypertrophy (D, arrows). The hypercellularity indicated by DAPI nuclear staining was found in the lesioned area of PION-induced instead of sham-treated optic nerve (E and F, arrow). Dramatic upregulation of ED1 staining is observed in the lesioned area in some animals 3 weeks after PION (G and H). Scale bar=100 μm.