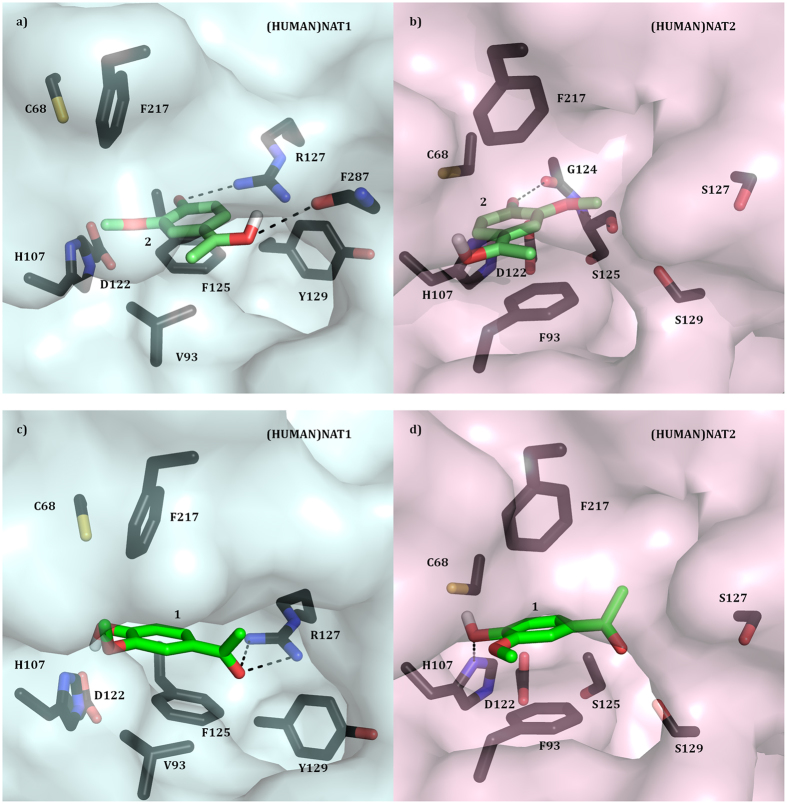
Figure 5. Substrate binding pockets of (HUMAN)NAT1 and (HUMAN)NAT2 with apocynin tautomers docked.
(a,b) The active site of (HUMAN)NAT1 (a) and (HUMAN)NAT2 (b)48 with docked apocynin quinonemethide tautomer (2) is shown in surface and stick representation respectively. The residues involved in ligand binding, substrate catalysis and substrate selectivity are shown in stick representation and labelled with carbon atoms in dark blue (a) or dark pink (b), nitrogen in blue, oxygen in red, and sulphur in orange. 2 is labelled with carbon atoms in green, oxygen in red, and hydrogen in white. (c,d) The active site of (HUMAN)NAT1 (c) and (HUMAN)NAT2 (d)48 with docked 1 is shown in surface and stick representation respectively. The residues involved in ligand binding, substrate catalysis and substrate selectivity are shown in stick representation and labelled with carbon atoms in dark blue (a) or dark pink (b), nitrogen in blue, oxygen in red, and sulphur in orange. 1 is labelled with carbon atoms in green, oxygen in red, and hydrogen in white.