Fig. 1.
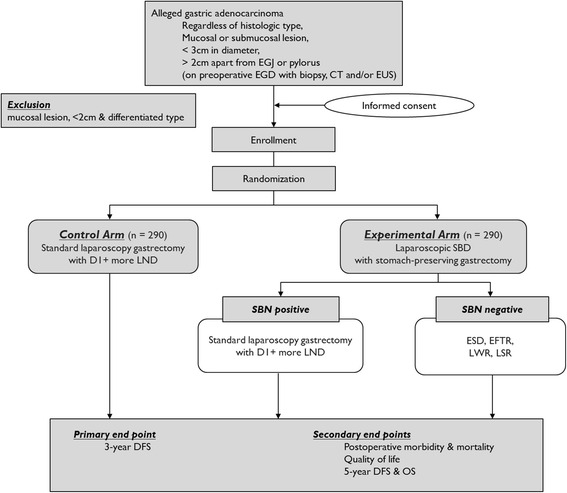
Study scheme of SENORITA trial, inclusion and exclusion criteria, intervention, and end points (EGJ, esophagogastric junction; EGD, esophagogastroduodenoscopy; CT, computed tomography; EUS, endoscopic ultrasonography; LND, lymph node dissection; SBD, sentinel basin dissection; SBN, sentinel basin node; ESD, endoscopic submucosal dissection; EFTR, endoscopic full-thickness resection; LWR, laparoscopic wedge resection; LSR, laparoscopic segmental resection; DFS, disease-free survival; OS, overall survival)
