FIG 4.
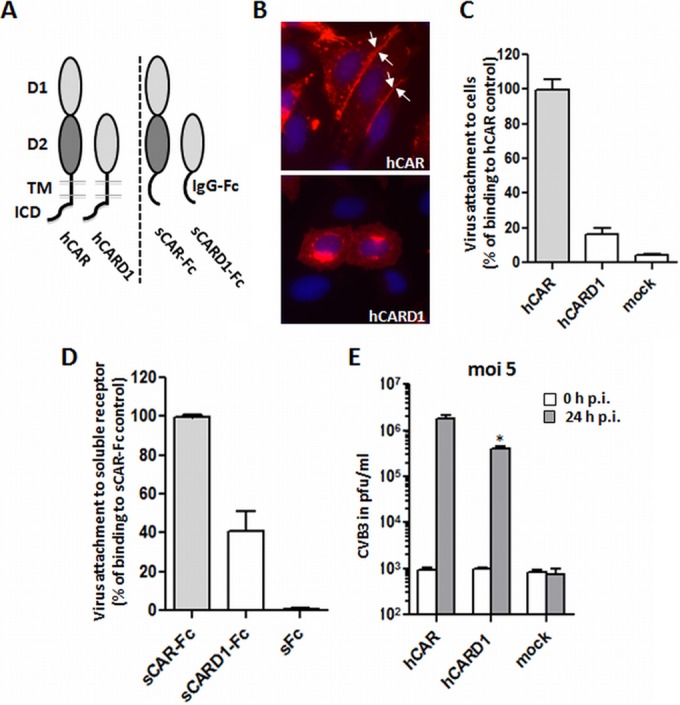
Deletion of CAR-D2 domain reduces, but does not prevent, virus attachment and infection. (A) Schematic illustration of wild-type hCAR and a D2 deletion mutant (hCARD1), expressed as cell surface receptors (left) and as dimeric soluble Fc fusion proteins (shown as monomers, right). hCAR consists of extracellular domains D1 and D2, a transmembrane domain (TMD), and an intracellular domain (ICD). In the deletion mutant hCARD1, the D1 domain is directly fused to the hCAR TMD. In the wild-type soluble fusion protein sCAR-Fc, the entire CAR ectodomain is fused to the immunoglobulin G Fc region; in the deletion mutant sCARD1-Fc, D1 alone is fused to Fc. (B) CAR localization in transfected CHO-K1 was analyzed by immunofluorescence using polyclonal anti-CAR antibodies. For the wild-type hCAR, the image was taken from Fig. 1. Wild-type hCAR was concentrated at cell-cell junctions; in contrast, hCARD1 showed little or no localization to junctions. (C) Binding of 35S-labeled CVB3 to CHO-K1 cells expressing hCAR or hCARD1, measured as described in Materials and Methods. The results are shown as means and the SD for three independent experiments. (D) Binding of 35S-labeled CVB3 to sCAR-Fc and the D2 deletion sCARD1-Fc mutant. Equal amounts of each soluble fusion protein (or sFc as a negative control) were bound to protein G-coupled magnetic beads, and virus binding was measured as described in Materials and Methods. (E) Viral replication in cells transfected with wild-type hCAR or hCARD1. Transfected CHO-K1 cells were incubated with CVB3 at an MOI of 5 for 1 h at room temperature, washed twice to remove unbound virus, and then incubated for 24 h at 37°C. Virus titers were determined by plaque assay at 0 and 24 h after infection. *, P < 0.05 (compared to hCAR control value).
