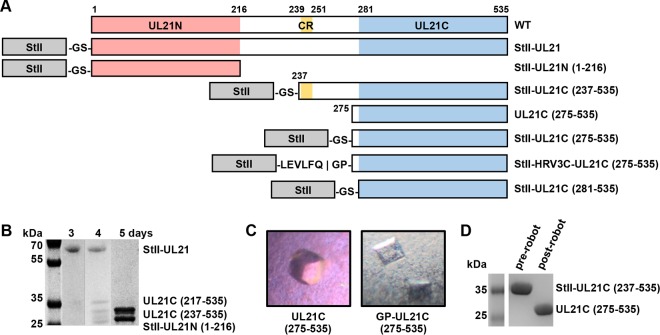
FIG 1.
Expression of UL21 constructs. (A) Linear diagrams of the predicted secondary structure and domains of UL21. Amino acid boundaries are indicated. WT, wild type; CR, the conserved region (residues 239 to 251) between UL21N (residues 1 to 216) and UL21C(281-535) that is predicted to form a β-strand. Constructs used in UL21C crystallization trials are also shown. (B) UL21 is proteolytically cleaved during purification by contaminating proteases. (C) UL21C crystals formed by spontaneous cleavage of UL21C during initial screening of crystallization conditions (left) or by cleavage due to an inserted protease cleavage site (right). (D) UL21C was cleaved during initial screening of crystallization conditions.