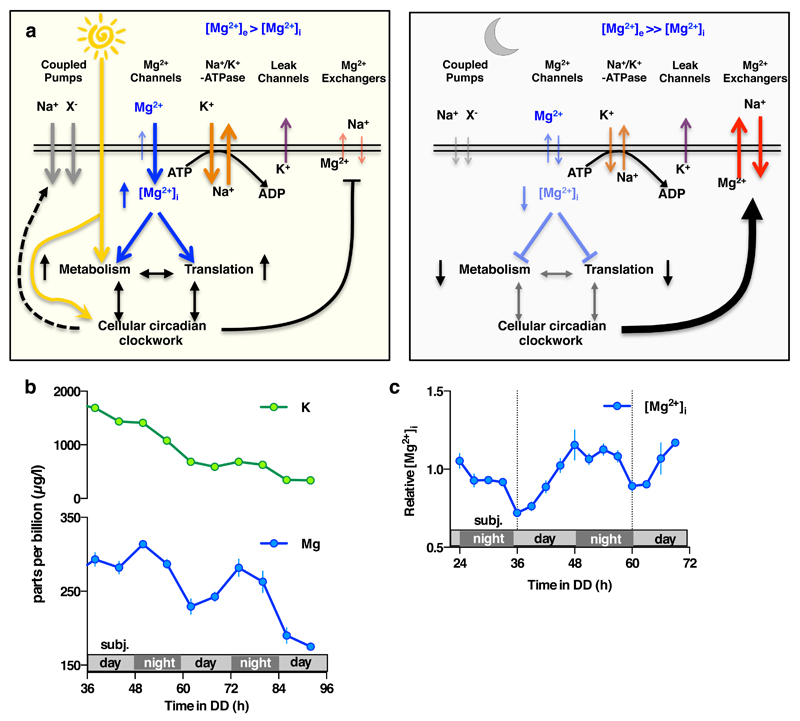
Extended Data Figure 10. Factors potentially contributing to maintenance of membrane electroneutrality in light of [Mg2+]i oscillations.
a. Model indicating potential ion fluxes that might explain how clock-regulated [Mg2+]i oscillations impact on global cellular metabolism whilst membrane electroneutrality is maintained, during the day versus the night. The observed phase dependency of acute CHA was different between Ostreococcus and U2OS cells (Fig. 4a-c), and is consistent with the very different environmental niches inhabited by a marine alga compared with a peripheral human tissue. In Ostreococcus, CHA maintained [Mg2+]i at daytime levels when added prior to the normal trough, resulting in increased nighttime translation and a concomitant reduction in relative ATP levels. This result suggests that Ostreococcus pumps magnesium out of the cell during the dark period, against a large electrochemical potential gradient (magnesium is the second most abundant cation in seawater, at 50 mM in this study) in order to globally down-tune ATP turnover. In U2OS cells, CHA treatment significantly reduced [Mg2+]i accumulation and translation rates as well as significantly increasing ATP levels when added prior to the [Mg2+]i peak. Human cells inhabit an environment where nutrient availability is homeostatically regulated (0.8 mM magnesium in cell culture medium). As such, circadian regulation of increased magnesium transport into the cell during the feeding, active phase of day serves to facilitate higher metabolic rate constants. Please note that light has no direct effect on the clock in human peripheral cells, instead being mediated by systemic cues. b-c. [Mg2+]i oscillations persist in transcriptionally inactive Ostreococcus cells kept in constant darkness, as analysed by both ICP-MS (b) and luciferase assay (c), indicating that circadian regulation of ion transport can occur post-translationally in addition to its transcriptional regulation (mean±SEM, n=3 for ICP-MS data and n=4 for luciferase assays).