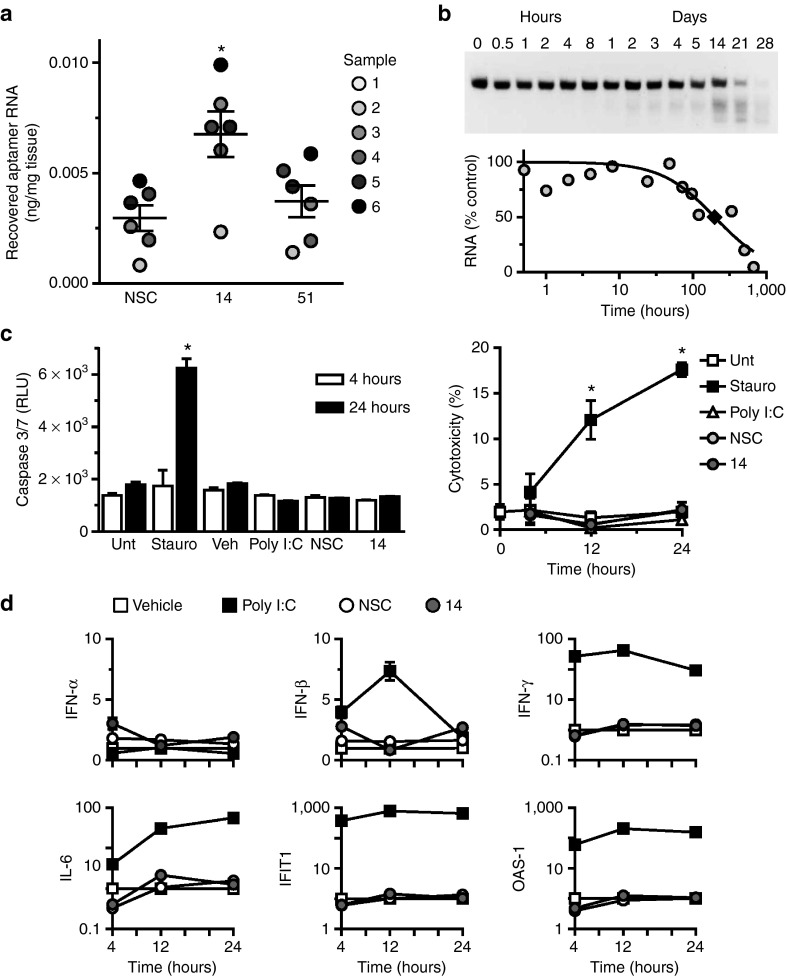
Figure 6.
Human cross-species reactivity, serum stability, and safety of aptamers. (a) Recovery of aptamers after incubation (150 nmol/l; 14, 51 or nonselected control (NSC)) with segments of human pulmonary artery, measured by RT-qPCR. Sample numbers refer to different patients as described in Supplementary Table S1. *P < 0.05 versus NSC. (b) Stability of Apt 14 (5 μmol/l) in 95% human serum. T1/2 = ~10 days (♦). (c) Assessment of aptamer (150 nmol/l; 14 or NSC) toxicity in human peripheral blood mononuclear cells. Measurement of Caspase 3/7 activation (left panel), measurement of lactate dehydrogenase (LDH) release (right panel). Percent cytotoxicity was determined by measuring LDH release as compared to maximum LDH release at each time point. *P < 0.05 versus untreated (Unt) for same time point. (d) Assessment of immunostimulatory effect of aptamers (150 nmol/l; 14 or NSC). Staurosporine: positive control for apoptosis/cytotoxicity; Poly I:C: positive control for immune stimulation. OAS-1, 2'-5' oligoadenylate synthetase 1A; INF-α, interferon-α; IFN-β, interferon-β1; IFN-γ: interferon-γ; IL-6, interleukin-6; IFIT1, interferon-induced protein with tetratricopeptide repeats 1. RT-qPCR, reverse-transcription quantitative PCR.