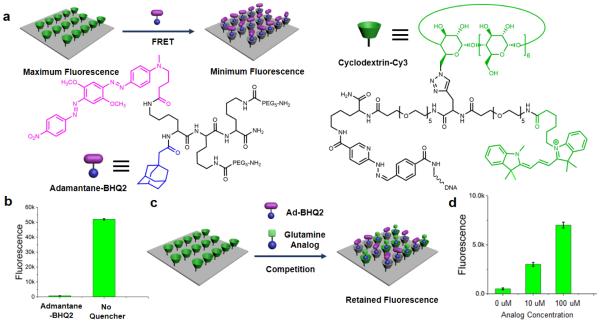
Fig. 1.
Illustration of the supramolecular glutamine assay. (a) The chemical structures and the functioning mechanism of the supramolecular FRET pair. The cyclodextrin-Cy3 was conjugated to a single strain DNA and then immobilized onto the glass slide through DNA hybridization. The Cy3 groups serve as FRET donor and the cyclodextrins as supramolecular host. A dark quencher group, BHQ2, was conjugated to an adamantane to form the FRET acceptor / supramolecular guest. The binding between adamantane and cyclodextrin brings BHQ2 to the vicinity of Cy3 and quenches the fluorescence. (b) Fluorescence intensities of the surface Cy3 with and without 100 nM of adamantane-BHQ2. (c) An adamantane-labeled glutamine analog competes with the adamantane-BHQ2 for the binding site of surface cyclodextrin, thus inhibiting the quenching process and retaining Cy3 fluorescence. (d) Fluorescence intensities of the surface Cy3 with 100 nM of adamantane-BHQ2 and different concentrations of the glutamine analog.