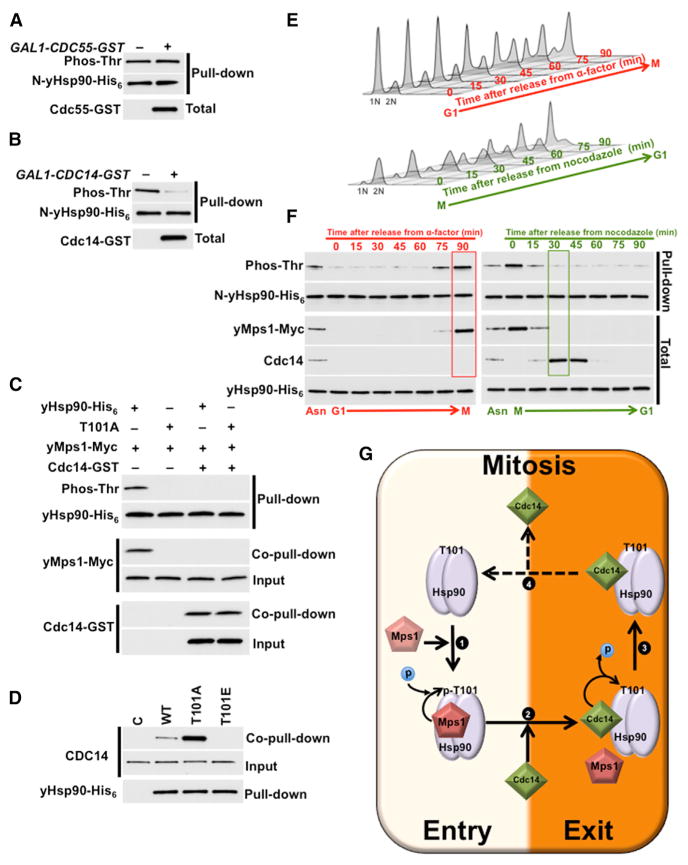
Figure 6. Cdc14 Phosphatase Targets yHsp90 and Dephosphorylates T101.
(A and B) Yeast expressing WT yHsp90-His6 (WT) and carrying (A) CDC55-GST or (B) CDC14-GST under GAL1-promoter were grown on YP-raffinose (−) overnight at 25°C and then shifted to YP-galactose (+) for 2 hr at 25°C. N-domain threonine phosphorylation of yHsp90 was assessed after pull-downs, PreScission protease digestion, and immunoblotting. Phos-Thr, phospho-threonine.
(C) yMps1-Myc was isolated from yeast, salt-stripped (0.5 M NaCl), and used to phosphorylate bacterially expressed and purified yHsp90-His6 (WT) and T101A-yHsp90-His6 (T101A). Cdc14-GST isolated from yeast, salt-stripped (0.5 M NaCl), and used to dephosphorylate T101. Total and phosphorylated yHsp90 was detected by immunoblotting.
(D) Yeast cells with yHsp90-His6 (WT), T101A-yHsp90-His6 (T101A), and T101E-yHsp90-His6 (T101E) mutants were isolated by Ni-NTA, and interaction of Cdc14 was examined by immunoblotting. Empty plasmid was used as a control (C).
(E) Yeast cells were released from G1 (2.5 μg/ml α-factor) to mitosis (20 μg/ml nocodazole) or from mitosis to G1 and then analyzed by flow cytometry every 15 min.
(F) Phosphorylation of the N-yHsp90-His6 (WT) from cells in (E) was analyzed by immunoblotting with pan-anti-phospho-threonine antibody. Cdc14, yMps1-Myc and full-length yHsp90-His6 protein levels were also detected by immunoblotting.
(G) Schematic representation of yMps1-mediated phosphorylation and Cdc14-facilitated dephosphorylation of T101-yHsp90. (1) yMps1 binds to yHsp90 and phosphorylates T101, and this promotes formation of yHsp90:yMps1 complex. (2) Cdc14 mediates dephosphorylation of T101, (3) disrupts yHsp90:yMps1 complex, and promotes yHsp90:Cdc14 association. (4) Possible dissociation of Cdc14 allows yMps1 binding and restarting the phosphorylation cycle.