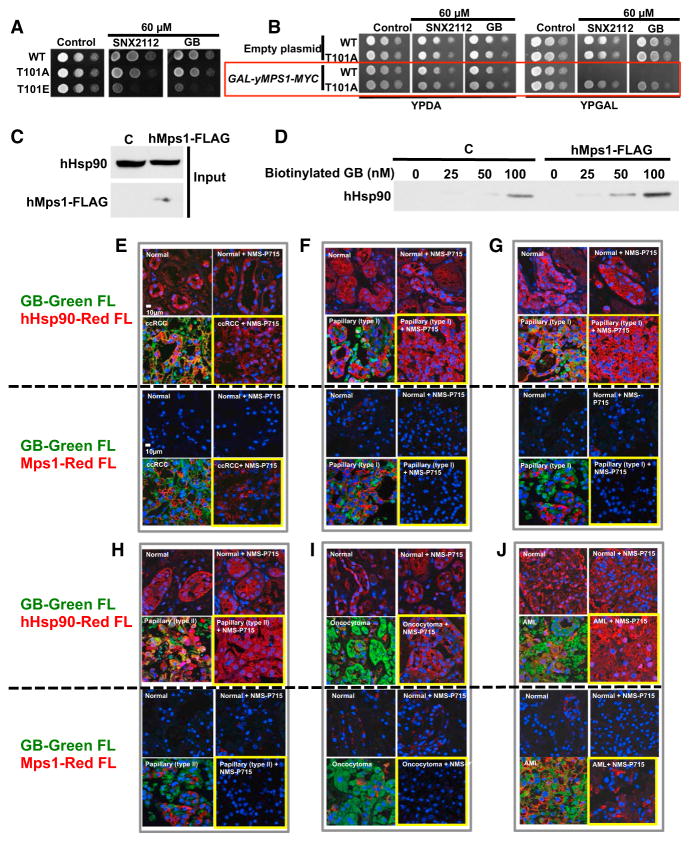
Figure 7. Mps1-Mediated Phosphorylation of Hsp90 Sensitizes Cells to Hsp90 Drugs and Confers Tumor Selectivity to Hsp90 Inhibitors.
(A) Yeast expressing yHsp90-His6 (WT), and indicated phospho-T101 mutants were spotted at a 1:10 dilution series of 107 cells per milliliter on YPDA agar containing 60 μM Hsp90 inhibitor SNX2112 or GB. Plates were incubated at 28°C for 4 days.
(B) Strains in (A) containing either empty plasmid (pYES2) or GAL-yMps1-MYC were grown on raffinose media overnight at 25°C. A 1:10 dilution series of 107 cells per ml were spotted on YPDA or YPGAL agar containing 60 μM SNX2112 or GB. Plates were incubated at 28°C for 4 days.
(C) HEK293 cells were transiently transfected with empty plasmid (C) or hMps1-FLAG. Protein expression was assessed by immunoblotting.
(D) Lysates from (C) were incubated with indicated amounts of biotinylated-GB, followed by streptavidin agarose beads, and Hsp90 was detected by immunoblotting.
(E–J) Adjacent normal tissues and (E) ccRCC , (F and G) papillary type I, (H) papillary type II, (I) on-cocytoma, and (J) AML were dissected into 3-mm3 pieces that were cultured in medium containing 10 μM NMS-P715 (Mps1 inhibitor) for 24 hr. Tissues were then incubated with 0.5 μM BODIPY fluorophore-conjugated GB (STA-12-9455; GB-Green-FL) for an additional 6 hr. Tissues were fixed and immunostained for either Hsp90 (hHsp90-Red FL), or hMps1 (hMps1-Red FL). DNA was stained with DAPI for immunofluorescence analysis.
See also Figure S4.