Fig. 5.
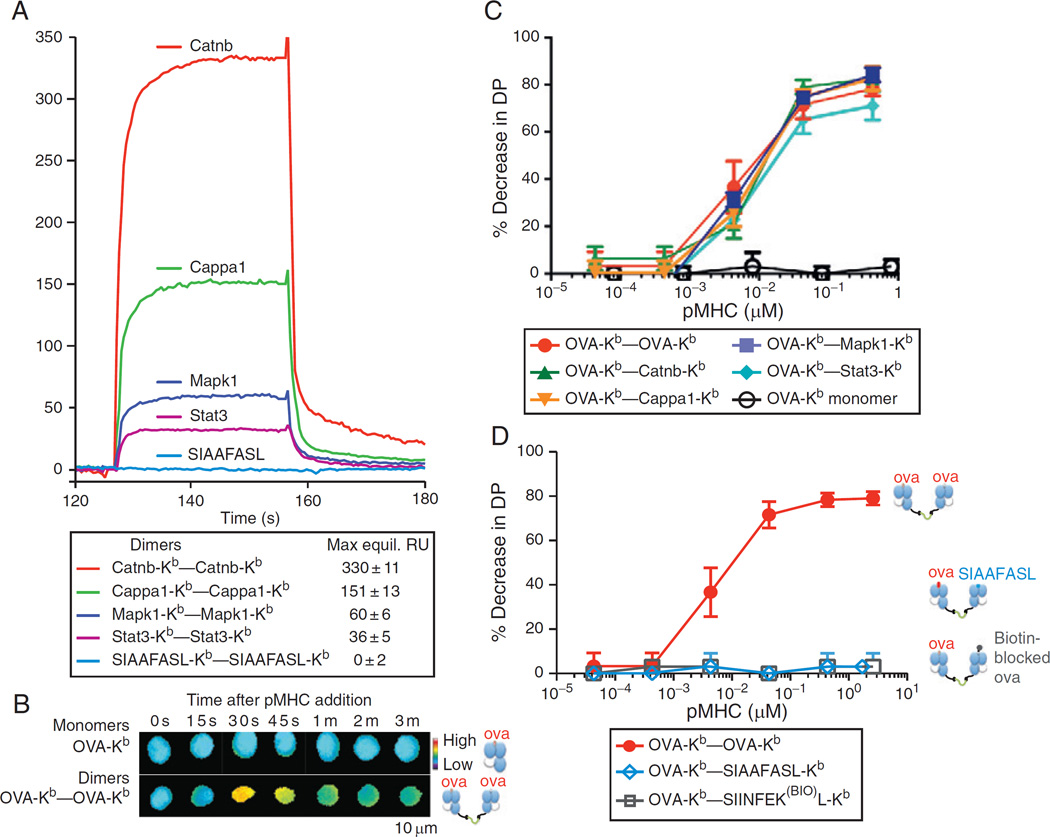
Self-peptide-MHC contributes to class I-restricted thymocyte signaling in a peptide-specific manner. (A) Surface Plasmon Resonance (SPR) measurement of binding of soluble dimeric complexes of the indicated self-peptide-Kbs to immobilized OT-I TCR. The Mapk1 and Stat3 self-peptide-Kbs bound OT-I with a measurable affinity, while the engineered “null” SIAAFASL-Kb did not. (B) Dimeric but not monomeric Ova-Kb drives calcium signaling by OT-I DP thymocytes. Thymocytes were taken from OT-I H-2K−/− H-2D−/− mice, loaded with the ratiometric calcium dye Fura2am, and exposed to high concentrations of soluble monomeric or dimeric Ova-Kb. Fura2 ratios (340/380 nm) are shown in a false-color scale. (C) Homodimeric or heterodimeric Ova-Kb, but not monomeric Ova-Kb, drives negative selection in whole thymi. E16 FTOC were established from OT-I H-2K−/− H-2D−/− mice, and the indicated soluble monomeric or dimeric H-2Kbs were added at varying concentrations. After 3–4 days, FTOC were disrupted, and the resulting thymocyte populations were assayed by FACS: the percentage of surviving DP thymocytes, relative to untreated control FTOCs, are shown. Ova-Kb monomers had no effect on thymocyte selection, even when added at concentrations above the KD of the Ova-Kb/OT-I interaction, where more than 50% of the thymocytes’ TCRs are expected to be bound by the Ova-Kb. Ova-Kb homodimers, or Ova-Kb–self-peptide-Kb heterodimers, could drive negative selection of the OT-I thymocytes to a similar extent, and at an equally low dose. (D) The ability of Ova-Kb–self-peptide-Kb to negatively select depends on the self-peptide-Kb’s affinity for the OT-I TCR. FTOC were established and assayed as in part (C). Negative selection did not result from even very high doses of the Ova-Kb–SIAAFASL-Kb (in which the peptide’s OT-I-contacting residues are mutated), or the Ova-Kb–SIINFEK(BIO)L-Kb (in which a primary predicted OT-I contacting residue in the Ova peptide, the p7 K, is modified with a biotin at the lysine’s free amine group). Data are originally from Ref. 38.