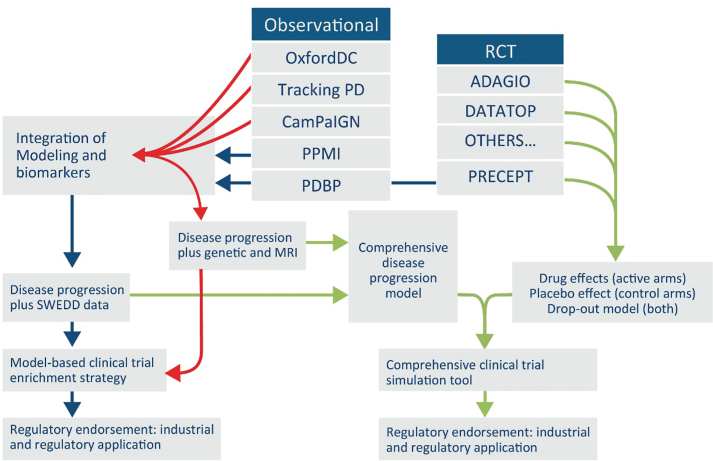
Fig.1.
Proposed Roadmap for building PD drug development tools with existing data. Proposed roadmap outlining a potential future path for integrating global data from PD observational and clinical trials targeting early stages. Integration of diverse data from at least seven independent clinical studies into a unified database will enable a regulatory path for use of biomarkers and quantitative disease progression models that serve to streamline and derisk drug development of new therapies.