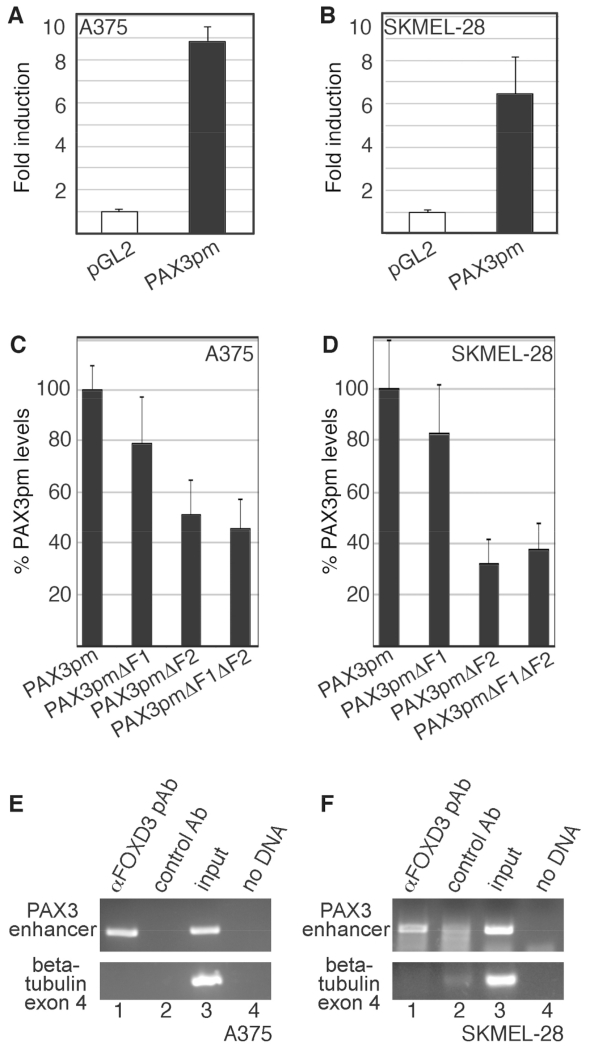
Fig. 6.
The PAX3 1.6kb proximal promoter is active in melanoma cells, and is bound to FOXD3 in melanoma cells. (A,B) A reporter construct containing 1.6 Kb of PAX3 promoter (PAX3pm) expresses luciferase in melanoma cells. pGL2-luciferase construct without (white bars) or with (black bars) a segment of the PAX3 promoter were transfected into A375 (A) and SKMEL-28 (B) cells. (C,D) The F1 and F2 PAX3 promoter sites are active in melanoma cells, with the F2 element being a major driver of expression. PAX3 promoter constructs with or without mutations in putative FOX sites were transfected into A375 and SKMEL-28 cells. For %PAX3pm levels, values were calculated by the measurement of arbitrary light units produced by each reporter divided by the PAX3pm levels, and multiplied by 100. For luciferase assays shown in A-D, each bar represents n=9, with standard error of the mean as shown. (E,F) FOXD3 is located on the endogenous PAX3 promoter in A375 (E) and SKMEL-28 (F) cells. Chromatin immunoprecipitation (ChIP) analysis was performed with primers specific for the PAX3 promoter region (top gels) or exon 4 of the beta tubulin gene (bottom gels, negative control). Proteins were precipitated with antibodies against FOXD3 (lane 1) or normal mouse IgG (negative control, lane 2). DNA from the cell lysate was tested as a positive PCR control (lane 3), and no DNA template as a negative control (lane 4).