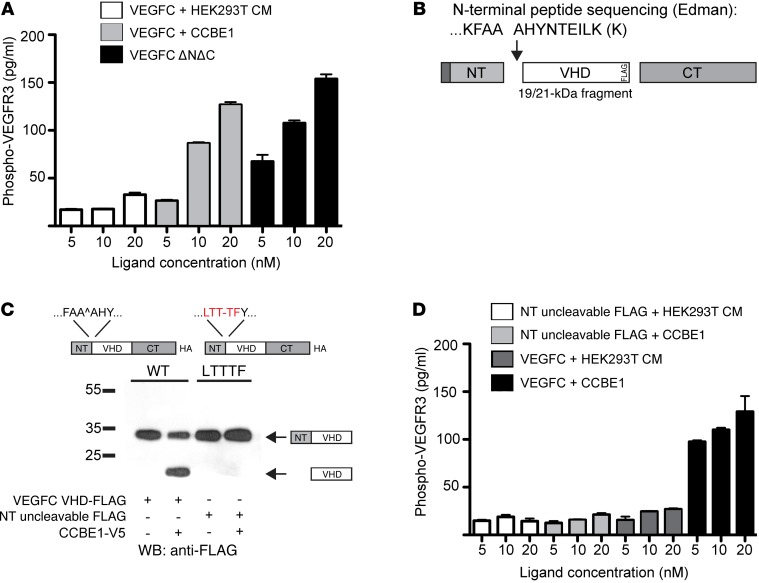
Figure 3. CCBE1-dependent proteolysis is required for VEGFC to activate VEGFR3 signaling.
(A) Phospho-VEGFR3 was measured by ELISA following LEC exposure to 5 to 20 nM VEGFC that was incubated with conditioned medium from control HEK293T cells (HEK293T CM) or CCBE1-V5 (CCBE1) or following exposure to VEGFC ΔNΔC-Fc. (B) Schematic representation of the VEGFC cleavage site generated following exposure to CCBE1 in HEK293T conditioned medium. (C) The VEGFC FAAAH109-113LTTTF mutant was not N-terminally cleaved in the presence of CCBE1. (D) N-terminally uncleavable VEGFC (NT uncleavable) was unable to activate VEGFR3. Phospho-VEGFR3 ELISA was performed as described in A using the indicated concentrations of WT and NT uncleavable VEGFC, with and without CCBE1. n = 3 for each concentration. Biochemical data shown are representative of 3 separate experiments. CM, conditioned medium.