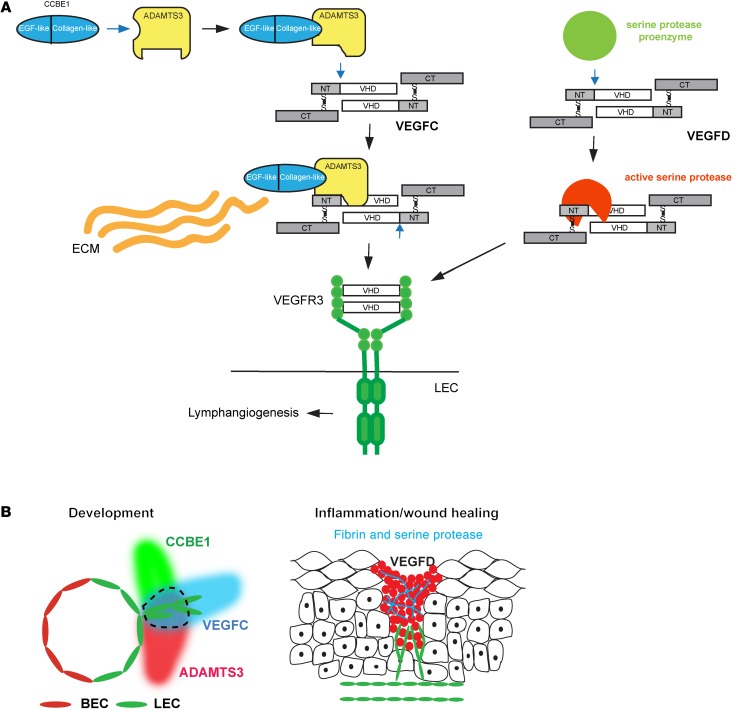
Figure 8. Distinct mechanisms of proteolytic activation support distinct biological roles for VEGFC and VEGFD.
(A) Molecular mechanisms of VEGFC and VEGFD activation. VEGFC is activated by the formation of a VEGFC-ADAMTS3-CCBE1 complex (left). CCBE1 binding to ADAMTS3 via the CCBE1 CT (left) is predicted to confer a conformational change that permits the enzyme to associate with and cleave VEGFC (middle). N-terminal cleavage of VEGFC releases the VHD that is able to activate VEGFR3 on the LEC. The CCBE1 NT may bind extracellular matrix (ECM) to localize the complex spatially during lymphatic growth. In contrast, VEGFD is activated independently of ADAMTS3 and CCBE1, most likely through a serine protease generated at sites of inflammation (right). (B) Proposed lymphangiogenic roles of VEGFC and VEGFD in vivo. VEGFC activation by ADAMTS3 and CCBE1 provides a mechanism for spatial patterning of the developing lymphatic vasculature (left). Schematic shows the mid-gestation cardinal vein with newly specified LECs that are in the process of sprouting to form the lymphatic network. The area encircled by the dotted line represents a zone of active VEGFC. VEGFD activation by inflammatory proteases such as those generated during wound healing in the skin provides a mechanism for lymphangiogenesis in mature animals with preexisting lymphatic networks (right).