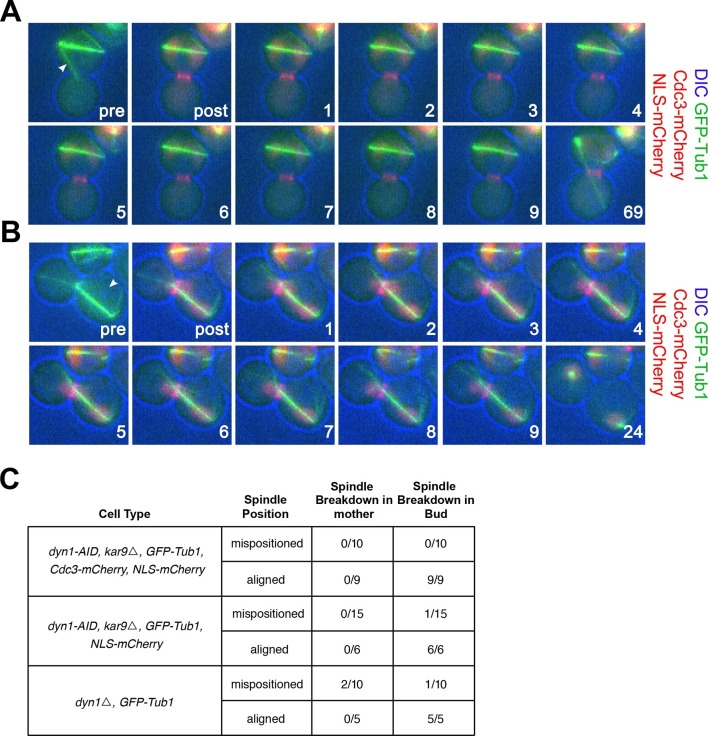
Figure 3. cMT laser ablation does not promote exit from mitosis in cells with mispositioned spindles.
(A-B) Cells harboring the osTIR1 DYN-AID kar9Δ constructs that also expressed GFP-tagged α-tubulin, mCherry-tagged Cdc3 and an NLS-mCherry (A35143) were grown overnight to mid-log in YEPD at 25°C. Cells were then resuspended in synthetic complete medium supplemented with 100 μM IAA and incubated for 2–3 hr at 25°C. The cells were prepared on an agar pad for live cell microscopy. Two pre-ablation images were taken (only one is shown) before the cMT was cut. Post ablation cells were monitored for 9 min at 1-min intervals for cMTs and then 1 hr at 15-min intervals to follow cell cycle progression. The arrowheads indicate the approximate laser targeting site. (A) A montage of a cell with a mispositioned spindle where cMT bud neck interactions were disrupted due to microtubule severing. The DIC channel is optimized to enhance contrast. (B) A montage of a control cell with an aligned spindle where the laser was targeted to the cytoplasm. The DIC channel is optimized to enhance contrast. (C) Table summarizing cell cycle stage of aligned and misaligned spindles 69 min post ablation. The culturing conditions for cells in the first (A35143) and second rows (A34832: the same as A35143 but lacking Cdc3-mCherry) of the table are the same as described above. In the third row, cells lacking DYN1 and expressing GFP-tagged α-tubulin and NLS-mCherry (A34722) were grown overnight at 25°C to mid-log and then shifted to 16°C for 2–5 hr to enrich for cells with mispositioned spindles. The cells were then mounted on an agar pad for live cell microscopy. One set of pre-ablation images was taken before the cMT was cut. Post ablation cells were monitored as described above.