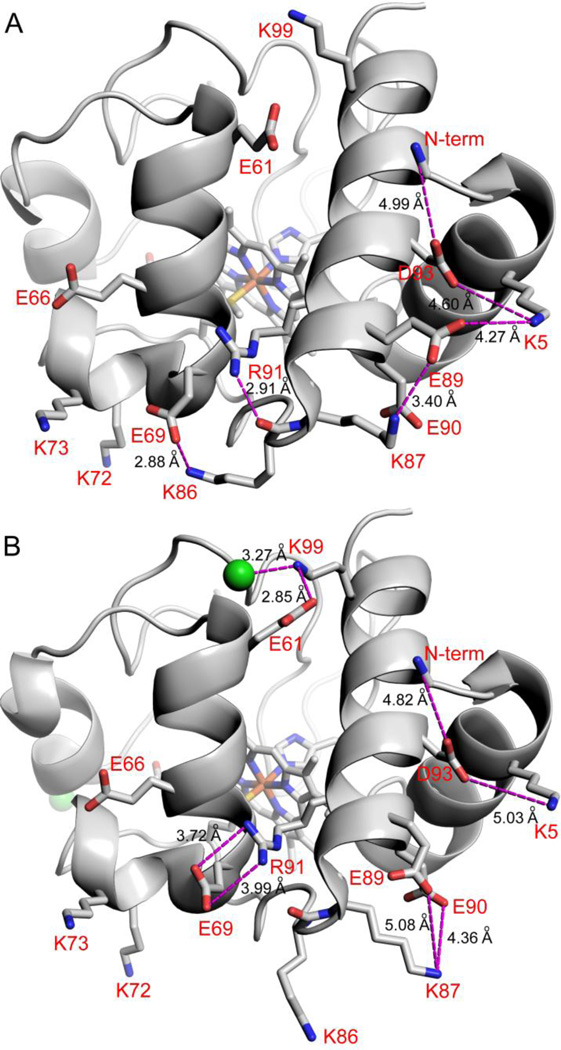
Fig. 6.
Effect of Cl− binding on electrostatic networks in SM Cytc. (A) Molecule B of SM Cytc (pdb code: 5DFS) showing electrostatic contacts (magenta dashed lines) of ~5 Å or less (distances given in black type) in the absence of Cl− binding. The dominant conformer (63% occupancy) of Arg91 is shown. The other conformer of Arg91 forms a shorter hydrogen bond of 2.72 Å to the main chain carbonyl of Lys86. (B) Molecule A of SM Cytc (pdb code: 5DFS) showing electrostatic contacts (magenta dashed lines) of ~5 Å or less (distances given in black type) in the presence of Cl− binding. Cl− ions are shown as green spheres. The dominant conformer of Arg91 (83% occupancy) is shown. The minor conformer of Arg91 forms a 2.78 Å hydrogen bond with the main chain carbonyl of Lys86. In both panels residues are labeled in red using one letter amino acids abbreviations and the N-terminal amino group is labeled N-term.