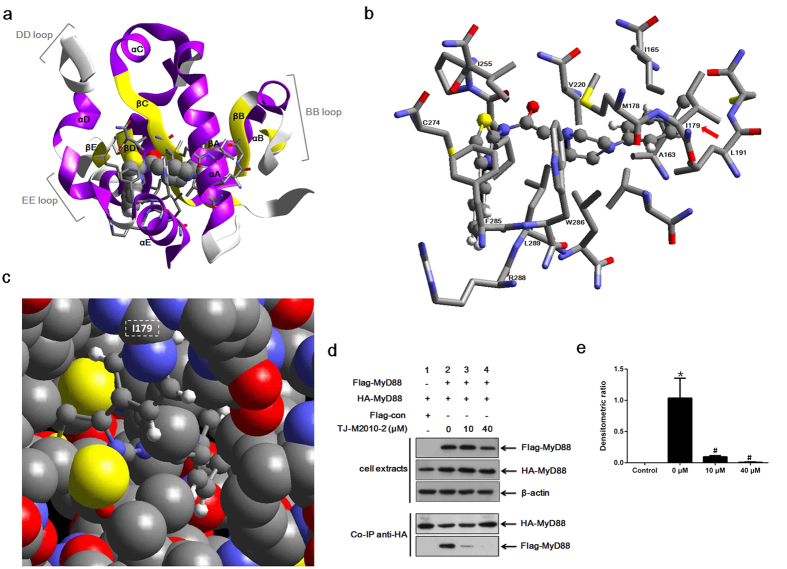
Figure 1. TJ-M2010-2 interacts with MyD88 TIR domain.
(a) The crystal structure of most TIR domains contains a five-stranded β-sheet (a–e) surrounded by five α-helices (a–e). TJ-M2010-2 embedded into the MyD88 TIR domain and interacting with the αA, αE, βC, βD, DD loop and EE loop. The MyD88 TIR domain crystal structure based on the Protein Data Bank (PDB ID: 4DOM). The non-bond interaction score was −747.325 kcal/mol. (b) TJ-M2010-2 acted on the indicated MyD88 TIR domain amino acid residues. The red arrow indicates the Poc residue site I179. (c) The dominating interacting site I179 is magnified and marked by the white rectangle with dotted lines. Gray: carbon atom; blue: nitrogen atom; red: oxygen atom; white: hydrogen atom; yellow: sulfur atom. (d) Co-immunoprecipitation assay (Co-IP) of MyD88. HEK293T cells were co-transfected with HA-MyD88/Flag-MyD88 or HA-MyD88/Flag-con. Seven hours after transfection, TJ-M2010-2 was added to the medium. Total protein was extracted 48 hours after transfection. Proteins were incubated with anti-HA antibody and Protein A + G Agarose, and Flag-MyD88 bound to HA-MyD88 was detected by an anti-Flag antibody. As the concentration of TJ-M2010-2 increased (0 μM, 10 μM, 40 μM), the binding capacity between the Flag-MyD88 and HA-MyD88 proteins decreased (Co-IP, lane 2, 3, 4) (one of three independent experiments). (e) Densitometric analysis of Co-IP assays. The inhibition of dimerization of Flag- and HA- MyD88 proteins was dose-dependent, as 91% inhibited at 10 μM TJ-M2010-2 and 99% inhibited at 40 μM compared to 0 μM. The density of each HA-MyD88 lane was divided by that of Flag-MyD88 (*p < 0.01 versus Control; #p < 0.01 versus 0 μM). Results are expressed as mean ± s.d. Control: lane 1 in Co-IP; 0 μM: lane 2 in Co-IP; 10 μM: lane 3 in Co-IP; 40 μM: lane 4 in Co-IP.